Preliminary Characterization and Haustorium Anatomy of Thonningia Sanguinea Vahl. (Balanophoraceae); a Cryptic Parasitic Species in Southern Nigeria
O Imarhiagbe1* and EI Aigbokhan2
1Department of Biological Science, Edo University Iyamho, Edo State, Nigeria
2 Department of Plant Biology and Biotechnology, University of Benin, Benin City, Nigeria
Submission: April 06, 2020; Published: May 14, 2020
*Corresponding author: O Imarhiagbe, Department of Biological Science, Edo University Iyamho, Edo State, Nigeria
How to cite this article: O Imarhiagbe, E Aigbokhan. Preliminary Characterization and Haustorium Anatomy of Thonningia Sanguinea Vahl. (Balanophoraceae); a Cryptic Parasitic Species in Southern Nigeria. JOJ Wildl Biodivers. 2020: 2(2): 555588 DOI: 10.19080/JOJWB.2020.02.555588
Abstract
The recognition of Thonningia sanguinea as a monotypic or polytypic species has been a subject of debate among plant taxonomists. Hence, to devise useful diagnostic and key distinguishing features, 18 morphological characters from 37 populations from various forested areas harbouring the parasite in Southern Nigeria were evaluated and subjected to a multifactorial analysis. Microanatomic features of the parasite-host interface were also examined. Results showed that the first 2 Principal components accounted for just 37% of the variability and as such were unable to explain much of the genetic variability among the populations. However, the dispersion and clustering pattern of various populations of T. sanguinea in the PCA scatter plot and the dendrogram showed some instances where subpopulations were distributed in different clusters. This indicates the possibility of an inherent variation within subpopulations. The host-parasite interface was observed to be of the complex type, comprising numerous composite bundles distributed in the gall (haustorium). In conclusion, T. sanguinea, morphologically, can be regarded as a monotypic species.
Keywords:Parasitic plant; Thonningia sanguinea; Cryptic; Morphology; PCA; Anatomy
Introduction
Thonningia sanguinea Vahl. (Balanophoraceae) is an endemic plant of tropical Africa that parasitizes forest tree species via a connection with their root [1,2]. It possesses a hypogenic growth habit, with a flowering stem that emerges from the ground to produce a bright red or pink inflorescence containing male and female flowers, distinguishable by presence or absence of pollens. The crowded flower heads are covered in leaves scales that lack chlorophyll. Below the ground, the plant ramifies in different directions, forming the haustorium (gall) at different points where it attaches to the roots of its host plants. Among ethnic groups in southern Nigeria, T. sanguinea is highly valued for medicine; the inflorescence-bract is used for treating skin infection, bronchial asthma, stomach upset sore throat and dysentery. The modified root and rhizome are used as appetite restorer and also as an aphrodisiac [3].
There is a dearth of information on the systematic relationships of the genus. Its cryptic occurrence in forest environments which has further made identification of variation among its species difficult. Ever since, the description of the plant, there has been a controversy on the identification of its species. The monotypic or polytypic nature of the plant is still in dispute. The defunct delimitation of Thonningia into 6 species have been faulted by some other authors who believe the characters employed, were those that would be expected to vary under different ecological conditions and hence, largely plastic in nature [4]. Among those previously recognized were: T. sanguinea Vahl, T. elegans Hemsl. T. dubia Hemsl. T. angolensis Hemsl. T. ugandensis Hemsl. T. sessilis Lecomte. T. malagasica Fawcett. The marked reduction of its general morphology is largely responsible for obscurity in detecting specific characters to identify the various species that possibly exist. Given the invaluable relevance of morphological characters in species description and the fact that T. sanguinea is widely used as medicine in the tropical region of Africa, proper identification of its available species becomes highly pertinent to devise a for proper taxonomic identification keys for the available species.
Furthermore, the nature of the host-parasite interface is yet to be made clear. Host-parasite interface in parasitic plants occur in two forms; simple or discrete and the complex type. The simple or discrete pattern of host interface occurs when the boundary of the two individuals is clearly defined. Beyond the discrete interface, there is no host tissue in the parasite’s body. This type of well-defined interface occurs in most hemiparasitic flowering plants such as mistletoes [5]. In the complex interface type, there is no discrete interface but rather a diffuse host/parasite interface. These interfaces are not restricted to the attachment site of the tuber of the host root, but instead, extend deep into the parasite tuber and are the major conducting strands in the parasite’s body as seen in Langsdorffia and Balanophora [5]. Thorough knowledge of the structure of cells and tissues is essential for an understanding of the host-parasite physiology [6]. Therefore, an anatomical investigation of the interface of connection in T. sanguinea becomes necessary to elucidate and give a proper understanding of the parasitic relationship with its various host species. Hence the study was designed to answer the following research questions:
(i) what diagnostic identification keys can be used to show morphological variations among accessions of T. sanguinea?
(ii) Can morphological characters help resolve the monotypic controversy of T. sanguinea
(iii) what unique anatomic features are present in the host-parasitic interface?
Materials and Methods
Study area
Forested areas harbouring T. sanguinea in Southern Nigeria were identified (Figure 1). These sites and their accompanying state and coordinates include Okomu National Park, Edo State (N 06024.113” 005019’.440” E), Cross River National Park, Cross River State (N 05021.863” 0080 26’.438’’E), Ofosu Forest Reserves, Ondo State (N 06043.278” 005007’.852’’E), Idanre Forest Reserves, Ondo State (N 07001.954” 005009’.868’’E), IITA Forest Reserves, Oyo State (N 07029.820” 003053’.530’’E), Oba Hills Forest Reserves, Osun State (N 07045.275” 004007’.752’’E), Ehor Nu Wire forest community, Edo State (N 06018.342” 0050 48’.598’’ E), Okuor community forest, Edo state (N 06011.962” 0060 04’.928’’E), Okokhuo community forest, Edo State (N 060 34.909” 0050 36.415” E), Iyanomo plantation forest, Edo State (N 060 09.746” 005034’.898’’E) and these areas were used as the data collection sites.

Sampling Strategy
Field survey was conducted in March 2017 to identify populations of T. sanguinea in its natural habitats (Figure 2). Because each population at different locations had different size class group, T. sanguinea inflorescence strand were grouped into different size-class based on the average diameter size of the mid-point of the inflorescence bud. This was necessary to standardize data collected. To ensure uniformity and avoid sampling bias, similar class-size groups common to the different locations were used to run the final data analysis. The various size-class measurements are shown in (Table 1).
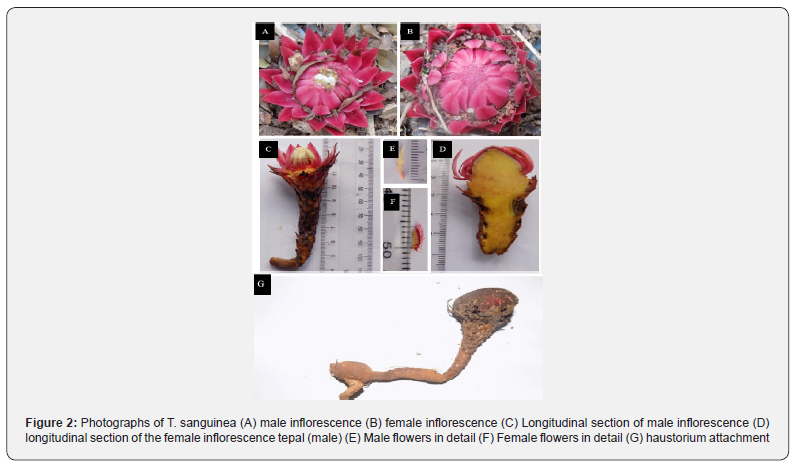

Data Collection
Qualitative and quantitative data were collected. Parameters measured were tuber diameter, inflorescence length, filament length, ovary length, anther length, style length, upper scale length, intermediate scale length, lower scale length, perianth segment, diameter of flower head, peduncle length, flower length, inflorescence scale shape, bract colour, tuber colour, nature of tip of bract, degree of tuber pubescence, sexuality). At least one herbarium specimen was prepared from each sample location and deposited at the Department of Plant Biology and Biotechnology Herbarium, University of Benin, Benin City.
Microanatomy features of the host-parasite interface
Anatomic features of the host-parasite interface (haustorium) in T. sanguinea were investigated using gall samples from three different host species, namely, Musanga sp, Lophira alata, Myrianthus sp were fixed in FAA (formalin: glacial acetic acid: 70% ethyl alcohol = 5: 5: 90 v/v) over 48 hours, then dehydrated in the following series (85%, 95%, 100% absolute ethanol), and then embedded in paraffin wax. Transverse serial sections were cut on a microtome at 10-15 mm and mounted on slides. Some sections were stained with safranine O. All sections were observed under a Zeiss light microscope and photographed with a mounted digital camera. Nomenclature and cell sizes were determined following microscopic terminology for hardwood identification [7].
Statistical Analysis
The multifactorial analysis was performed to assess the possible relationship between qualitative and quantitative morphological characters and populations. Data collected were analyzed using the PAST (Palaeontological Statistics) Ver. 1.43 software. Principal Component Analysis (PCA) was used to determine the extent of genetic variation and the percentage similarities within and between the populations. Eigen-values and principal component scores were obtained using the PCA; these were then used to determine the relative discriminative power of the axes and their associated characters. The pattern of relationship among the populations was further illustrated by a scatter plot and a dendrogram using the Neighbour-joining algorithm.
Result
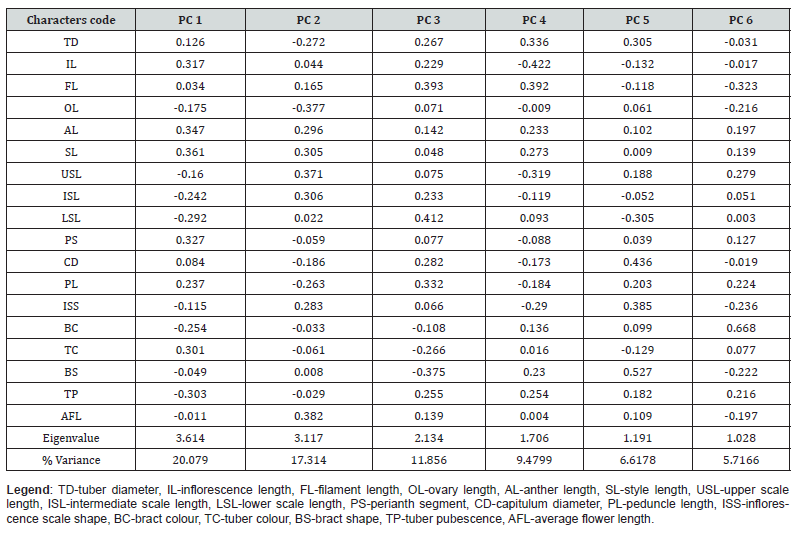
The results are presented in (Table 2) and (Figure 2). The Principal Component Analysis conducted for 18 morphological characters among 37 populations of T. sanguinea showed that the total variation of the PCA1 and PCA2 (37%) cannot explain the morphological variation. However, an accumulated percentage variance of the first 6 principal components gave a value of 71.04 %. The Eigen-value of the first 6 (six) principal components as well as their accompanying percentage variance are as follows: PCA 1 (3.61; 20.08 %), PCA 2 (3.12; 17.31 %), PCA 3 (2.13; 11.86 %), PCA 4 (1.71; 9.48 %), PCA 5 (1.19; 6.62 %) and PCA 6 (1.03; 5.72%). The first 6 (six) of the 37 principal component axes had Eigen-values greater than 0.7 as per the Joliffe cut off standard value. The Eigen-values tell much about the importance of each principal component axes and their contributions in explaining the variability in the morphological characters of the 38 populations of T. sanguinea collected across Southern Nigeria. This clearly shows that the first two principal axes do not accrue enough percentage variance to adequately explain the genetic variations within the populations of T. sanguinea (Table 2). The loads’ values of 18 morphological characters on the different principal component axis were used to ascertain the relative contribution of each in explaining the variability among the 37 accessions of T. sanguinea. Characters with relative high loads on the PCA 1 include: IL (0.316), AL (0.341), PS (0.327), and TC (0.331). Characters with relative high loads on the PCA 2 include: SL (0.305), ISL (0.306), and FL (0.381). Characters with relatively high loads on the PCA 3 include FL (0.344) and CD (0.332).
Table 2: Principal Component analysis of the 18 morphological characters of 37 Populations of Thonningia sanguinea
(Table 3) shows the principal component scores of the different accessions of T. sanguinea. The principal component scores indicate the scores of each T. sanguinea accessions on the different principal component axis. Accessions from Ehor Nu wire community forest, Okomu National Park and IITA forest reserves were best described by characters that were significantly loaded on PCA I. These characters include inflorescence length, anther length, perianth segment and, tuber colour. T. sanguinea populations from Okuor Forest and Cross River National Park were best described by characters that were significantly loaded on PCA 2. These characters include style length, Intermediate scale length, and flower length. T. sanguinea populations from IITA forest were specifically more described by characters that were significantly loaded on PCA 3. These characters include filament length and diameter of the flower head.

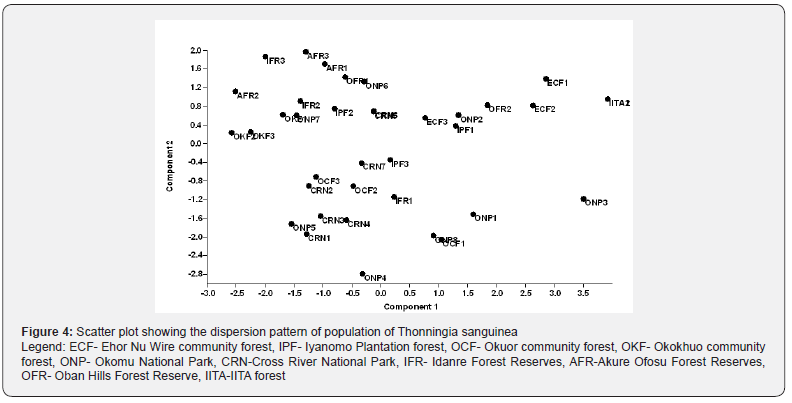
To complement the PCA results, a dendrogram of T. sanguinea populations from selected sites in Southern Nigeria was constructed using the Neighbour joining algorithm (Figure 3). The dendrogram reveals an irregular clustering pattern among the different populations and subpopulations. However, there seems to be an inherent heterogeneity pattern among the populations that probably cannot be fully explained using morphological data. The scatter plot again showed a dispersion pattern of the populations of T. sanguinea using morphological data. This further confirms the inherent variability among subpopulations (Figure 4). For instance, populations from the Okomu National Park had a representative in three different clusters of the dendrogram.


(Figure 5) shows the light micrographs of the interface between T. sanguinea and three (3) of its host species in Southern Nigeria, namely Musanga sp, Lophira alata, Myrianthus sp. Each of the parasite-host association shows a similar distribution of cells within the area of the interface. Generally, the anatomy of the parasite- host interface reveals the following features: H- host vascular tissue; G- gall (Haustorium) cells; T-transfer cells; C- collapsed tuber matrix; B- composite bundle sheath, M-Parasite/host meristems. Each interface unit consist of a composite bundle sheath, made up of transfer cell of the parasite intermixed with the host cell vascular tissue. Another notable feature is the collapse tuber matrix zone, which occurs as a result of the differential growth pattern of the vascular tissues of the parasite and host. An overview of the haustorium indicated that the vascular tissues of the host root ramify, along with parasite tissues, into the tuber matrix and formed an intertwined network of host/parasite interface. However, there was no evidence of discrete vascular connectivity, but rather a complex aggregate of composite bundles distributed within the haustorium matrix cells of the parasite (T. sanguinea).
Discussion
The use of the traditional morphological marker as a means of species delimitation comes with a lot of drawbacks, some of these include, phenotypic plasticity, character accessibility and difficulties with identifying intra-specific morphological variations especially among cryptic species. However, morphological characterization is still much relevant for rapid field species recognition and sampling. The present study attempts to identify key morphological characters to help differentiate among the various accessions of T. sanguinea. The results revealed that none of the principal component axes had significantly high enough Eigenvalue to completely explain the variability among the population of T. sanguinea in Southern Nigeria. Principal component analysis PCA 1 and PCA2 could only explain just 37 % of the variability in the population; notwithstanding, T. sanguinea populations from Ehor Nu Wire Forest, Okomu National Park, and IITA forest were best described by characters such as Inflorescence length, anther length, perianth segment, and tuber colour. On the other hand, T. sanguinea populations from Okuor forest and Cross River National Park were best described by characters such as Style length, Intermediate scale length, and Flower length.
Morphologically, T. sanguinea can be seen as a monotypic taxon due to the low morphological variability that exists among different populations across Southern Nigeria. The dispersion and clustering pattern of T. sanguinea populations in the PCA scatter plot and the dendrogram, however, showed some instances where subpopulations were distributed in different clusters. This indicates the possibility of an inherent variation within the different population in Southern Nigeria. Molecular methods have proved efficacious in detecting genetic differences among possible intra- species where the morphological marker has failed [8]. In the present case of T. sanguinea, the use of molecular markers such as RAPD, AFLP, or SSR is could proffer better insights on the cryptic status of the plant, especially within a population.
The anatomy of the host-parasite interface as examined from the study revealed a unique ground tissues composed predominantly of a mixture of parenchymatous cells from the host and parasite. The presence of such parenchyma cells at the point of interface signifies that the haustorium divides continuously. Such continuous cell division put the parasite in an advantage position to acquire maximum nutrient. In contrast to that of the host, the parenchymatous cells of T. sanguinea were deeply stained as reflected in the degree of the dye absorbance. This discrepancy in the degree of dye absorbance could have some metabolic implications and according to Hsiao et al. [5], the presence of large numerous heterochromatic nuclei in the parasite tissue could have an evolutionary advantage to drive higher metabolic rate of material migration from host to parasite and this was responsible for the deep dark red stain with safranin O dye.
Essentially, the anatomy of the parasite-host interface showed no evidence of discrete vascular connectivity but was composed of a complex aggregate of composite bundles scattered within the haustorium matrix. The host-parasite interface in T. sanguinea was of the complex type, made up of a diffuse host/parasite connection, comprising numerous composite bundles. Composite bundles are the differentiated cells of the interface of the parasite and host root. It forms a branching network that extends from the host plant root deep into the tuber (Gall). Within the core of the interface of the association, lie the intrusive cells of T. sanguinea that are believed to play an active role in the transfer of materials from the host xylem to the parasite.
Having a host/parasite interface consisting purely of transfer cells is unusual; the most common mode of parasitism falls into a simple and discrete host/parasite attachment [9]. At such a discrete host/parasite interface, there are direct contacts of parasite parenchyma with host phloem or direct xylem-xylem contacts. However, such xylem-xylem contacts never occur in the composite bundles of most members of Balanophracaeae [5]. The structure of the host/parasite interface deserves special attention because it is where enzymatic actions take place during the intrusive phase and also, where water and nutrient are transferred in the conductive phase. In some cases, it has been known to be involved in defense actions performed by the host. One of such actions is the development of tyloses previously observed by Idu et al. [7]. The diffuse host/parasite connection in the composite bundles present in members of Balanophoraceae appears to be a rare mode of parasitism. There are possibilities that this mode of contact might have evolved to enable holoparasites to siphon more from the solely dependable host species [10].
Conclusion
The foregoing study investigated the monotypic or polytypic nature of T. sanguinea in southern Nigeria using morphological marker. As inferred, T. sanguinea is morphologically an inseparable species and therefore, should be treated as monotypic. The host-parasite interface was observed to be of the complex type, comprising numerous composite bundles distributed in the gall (Haustorium). This finding further affirms T. sanguinea close relationship with Langsdorffia and Balanophora in terms of the nature of the parasite-host interface.
Acknowledgements
This work was completely funded by the TETFUND 2017- 2018 (Batch 12th) Research Project (RP) Intervention. The authors also wish to acknowledge the support of Rangers of the different National Parks and Forest Reverses visited, for their role in field sampling and data collections.
References
- Bullock AA (1948) Thonningia Vahl. Kew Bulletin, 3(3): 363.
- Duminil J, Di Michele M (2009) Plant species delimitation: A comparison of morphological and molecular markers. Plant Biosystems 143(3): 528-542.
- Glatzel G, Geils BW (2009) Mistletoe ecophysiology: host–parasite interactions. Botany 87(1): 10-15.
- Goto R, G Yamakoshi, Matsuzawa T (2011) A novel brood-site pollination mutualism?: the root holoparasite Thonningia sanguinea (Balanophoraceae) and an inflorescence-feeding fly in the tropical rainforests of West Africa. Plant Species Biology 27(2):164–169.
- Hsiao SC, Mauseth JD, Gomez LD (1993) Growth and Anatomy of the Vegetative Body of the Parasitic Angiosperm Helosis cayennensis (Balanophoraceae). Bulletin of the Torrey Botanical Club 120(3): 295-309.
- Hsiao SC, Mauseth JD, Peng CI (1995) Composite bundles, the host/parasite interface in the holoparasitic angiosperms Langsdorffia and Balanophora (Balanophoraceae). American Journal of Botany 82(1): 81-91.
- Idu M, Begho ER, Akpaja EO (2002) Anatomy of attachment of the root parasite. Indian Journal of Natural Rubber Research 15(1): 33-35.
- Imarhiagbe O, Aigbokhan EI (2019) Studies on Thonningia sanguinea Vahl. (Balanophoraceae) In Southern Nigeria: Range and Host Preference. International Journal of Conservation Science International Journal of Conservation Science 10(4): 721-732.
- Imarhiagbe O (2020) Prospects of Ethnobotanical Uses of Thonningia sanguinea Vahl. (Balanophoraceae) Among Selected Tribes in Southern Nigeria. Journal of medicinal plant studies.
- White DJB, Wheeler EA, Baas P, Gasson PE (1991) IAWA List of Microscopic Features for Hardwood Identification by an IAWA Committee. Kew Bulletin, 46(2): 376.






























