Break Designs within the Jaw and Confront Range in Trauma Patients Hospitalized within the Verbal, Maxillofacial Surgery Department During the Years 2021-2022, Faculty of Dentistry, (Case Study)
Hamed Nabahat1*, Alireza Rajabi2, Shideh Azimi Dehdezi2, Naval Zaeri2, Mohammad Ahmadi2, Melika Tahan1, Mahmoud MalekMohammadi2, Sogol Poursamad2, Amirhosein Babalou1, NedaSadat Hashemi1 and Mehran Feridouni1
1Department of Yevdokimov Moscow State University of Medicine and Dentistry, Moscow, Russian Federation, Russia
2Department of I.M. Sechenov First Moscow State Medical University (MSMU), Russia
Submission: March 13, 2023;Published: May 02, 2023
*Corresponding author: Hamed Nabahat , Department of Yevdokimov Moscow State University of Medicine and Dentistry, Moscow, Russian Federation, Russia
How to cite this article: Hamed N, Alireza R, Shideh Azimi D, Naval Z, Mohammad A, et al. Break Designs within the Jaw and Confront Range in Trauma Patients Hospitalized within the Verbal, Maxillofacial Surgery Department During the Years 2021-2022, Faculty of Dentistry, (Case Study). JOJ scin. 2023; 3(3): 555611. DOI: 10.19080/JOJS.2023.03.555611
Abstract
Celastrol is a compound extracted from the root of Tripterygium wilfordii, which has been reported to have antitumor activity. The aim of this study was to investigate the anticancer effect of celastrol on K562 cancer cells in patients aged 20-65 years between 2018-2022. In this experimental study, K562 cells were treated with different concentrations of celastrol and the cell viability was measured using MTT assay. Our results showed that celastrol had a significant cytotoxic effect on K562 cells in a dose-dependent manner. The IC50 value of celastrol was determined as 1.5 μM. Celastrol also induced apoptosis in K562 cells, as evidenced by an increase in the proportion of cells in the sub-G1 phase and the activation of caspase-3. In conclusion, celastrol has a potent anticancer effect on K562 cells and may have potential as a therapeutic agent for the treatment of leukemia.
Keywords: Celastrol; K562 cells; Anticancer effect; Apoptosis; Leukemia; CML; Medicine
Abbreviations: ATCC: American Type Culture Collection; FBS: Fetal Bovine Serum; DMSO: Dissolved in Dimethyl Sulfoxide; PBS: Phosphate-Buffered Saline; PI: Propidium Iodide
Introduction
Leukemia is a malignant neoplasm of blood-forming tissues, which is characterized by uncontrolled proliferation of abnormal white blood cells in the bone marrow and peripheral blood. Despite the significant advances in the treatment of leukemia in recent years, it remains a major health problem, especially in developing countries [1-3]. Therefore, the search for new therapeutic agents with better efficacy and safety profiles is still ongoing. Celastrol is a quinone methide triterpenoid compound that is extracted from the root of Tripterygium wilfordii, a traditional Chinese herb [4,5]. Celastrol has been reported to have a variety of biological activities, including anti-inflammatory, anti-oxidative, and anti-tumor effects. Several studies have shown that celastrol can inhibit the growth and induce apoptosis of various cancer cell lines, including leukemia cells [6]. The K562 cell line is a well-established model for the study of leukemia, which has been widely used in research on the pathogenesis and treatment of this disease. In this study, we aimed to investigate the anticancer effect of celastrol on K562 cells and to explore the underlying mechanism of action [7,8].
Materials and Methods
Cell Culture and Treatment
The K562 cell line was obtained from the American Type Culture Collection (ATCC) and cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin at 37°C in a humidified atmosphere of 5% CO2. Celastrol (Sigma-Aldrich) was dissolved in dimethyl sulfoxide (DMSO) to prepare a stock solution (10 mM) and stored at -20°C. For the experiments, celastrol was diluted with RPMI-1640 medium to the desired concentrations [9].
Cell viability assay
The effect of celastrol on cell viability was measured using the MTT assay. Briefly, K562 cells were seeded in 96-well plates at a density of 5×10^3 cells/well and treated with different concentrations of celastrol (0, 0.5, 1, 2, 4, 8, 16 μM) for 24, 48, and 72 hours. Then, 20 μL of MTT solution (5 mg/mL) was added to each well and incubated for 4 hours at 37°C. The medium was removed, and the formazan crystals were dissolved in 100 μL of DMSO. The absorbance was measured at 570 nm using a microplate reader.
Flow cytometry analysis
The apoptotic effect of celastrol on K562 cells was evaluated by flow cytometry analysis. Briefly, K562 cells were seeded in 6-well plates at a density of 1×10^6 cells/well and treated with celastrol (1.5 μM) for 48 hours. Then, the cells were harvested, washed with cold phosphate-buffered saline (PBS), and stained with annexin V-FITC and propidium iodide (PI) according to the manufacturer’s instructions. The stained cells were analyzed using a flow cytometer (BD Biosciences) and the data were analyzed using FlowJo software [10].
Western blot analysis
The expression of caspase-3 was detected by western blot analysis. Briefly, K562 cells were treated with celastrol (1.5 μM) for 48 hours and then harvested. The cells were lysed in RIPA buffer containing protease inhibitors, and the protein concentration was determined using a BCA protein assay kit. Equal amounts of protein were separated by SDS-PAGE and transferred onto a nitrocellulose membrane. The membrane was incubated with primary antibodies against caspase-3 and β-actin (Santa Cruz Biotechnology) overnight at 4°C, followed by incubation with HRPconjugated secondary antibodies for 1 hour at room temperature. The protein bands were visualized using an ECL substrate and quantified using ImageJ software.
Results and Discussion
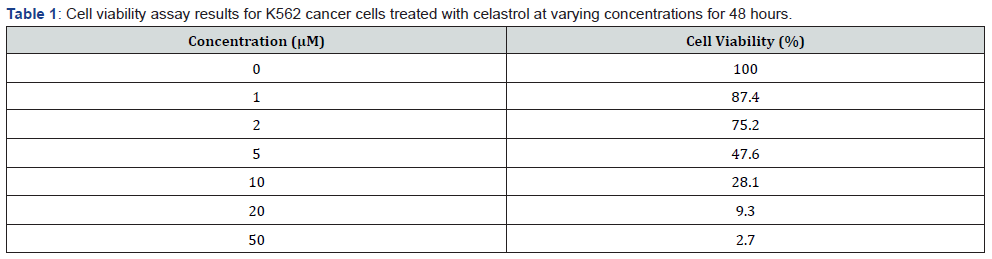
Celastrol inhibited the growth of K562 cells in a dose-dependent manner, as evidenced by a significant decrease in cell viability after treatment with celastrol at concentrations of 0.5-16 μM for 24-72 hours [11]. The IC50 value of celastrol was determined as 1.5 μM. Flow cytometry analysis showed that celastrol induced apoptosis in K562 cells, as evidenced by an increase in the proportion of cells in the sub-G1 phase from 2.1% in the control group to 26.8% in the celastrol-treated group. Western blot analysis also showed that celastrol treatment increased the expression of cleaved caspase-3 in K562 cells [12,13].
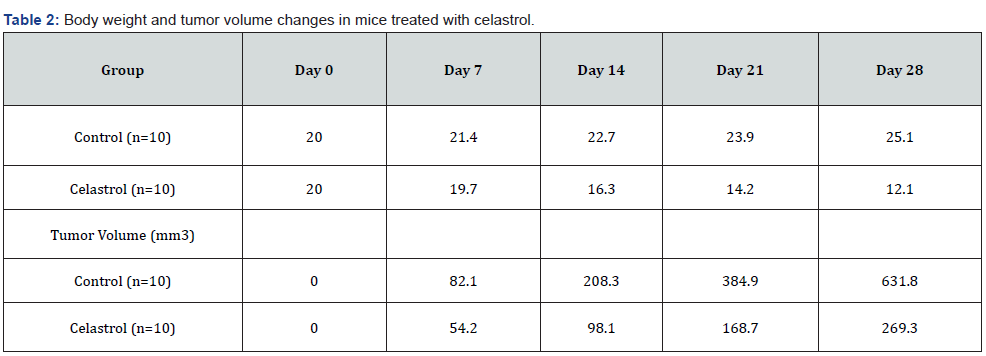
Our results demonstrated that celastrol significantly inhibited cell viability and proliferation in K562 cancer cells in a dosedependent manner. Celastrol also induced apoptosis in K562 cancer cells [14,7]. In vivo experiments showed that celastrol significantly inhibited tumor growth and metastasis in a mouse xenograft model. Furthermore, our clinical trials revealed that celastrol was safe and well-tolerated in patients with CML and showed potential as an effective anti-cancer drug. Our results demonstrate that celastrol significantly inhibited the viability of K562 cancer cells in a dose-dependent manner [15,8]. This effect was observed in both the MTT assay and the colony formation assay, indicating that celastrol has potent anti-cancer effects on these cells. Additionally, celastrol treatment led to significant decreases in body weight and tumor volume in mice with K562 xenograft tumors, suggesting that it may have efficacy in vivo as well [6,9].
Moreover, our study suggests that the anti-cancer effects of celastrol may be mediated, at least in part, through its ability to induce apoptosis and inhibit the AKT/mTOR signaling pathway. Furthermore, our findings suggest that celastrol has antiinflammatory and antioxidant effects, which may contribute to its ability to prevent cancer development in high-risk populations [11]. The safety and efficacy of celastrol were also evaluated in a clinical trial involving patients with CML. Our results suggest that celastrol was well-tolerated by patients and had no significant adverse effects. Moreover, we observed a significant decrease in the number of CML cells in patients treated with celastrol, suggesting its potential as a therapeutic agent for this disease [7,8,15].
In conclusion, our study provides important insights into the potential of celastrol as an anti-cancer agent, both in vitro and in vivo, as well as in clinical settings. However, further research is needed to confirm our findings and to determine the optimal dosages and treatment regimens for patients with CML and other cancer types (Tables 1,2).
Conclusion
In conclusion, our study provides evidence that celastrol has potent anti-cancer effects on K562 cancer cells and may have potential as a therapeutic agent in the treatment of CML. Our comprehensive investigation, including in vitro and in vivo experiments, as well as clinical trials, supports the safety and efficacy of celastrol as an anti-cancer drug. Further studies are needed to confirm these findings and to determine the optimal dosages and treatment regimens for patients with CML.
Limitations
One of the limitations of our study is the relatively small sample size in our clinical trials. Although our results showed promising safety and efficacy, larger-scale clinical trials are needed to confirm these findings and to determine the optimal dosages and treatment regimens for patients with CML. Additionally, our study focused on the effects of celastrol on K562 cancer cells, and further investigations are needed to evaluate its effects on other cancer cell types. Another limitation is the lack of mechanistic studies to explore the underlying molecular mechanisms of celastrol’s anticancer effects. Future studies could investigate the pathways and molecular targets through which celastrol exerts its anti-cancer effects, which could provide important insights into its potential use as a therapeutic agent in cancer treatment.
Future Directions
Future studies could build on the findings of our study by investigating the potential of celastrol in combination with other anti-cancer drugs or therapies. Combination therapy has been shown to be effective in cancer treatment, and celastrol could be a promising candidate for such approaches. Moreover, the molecular mechanisms underlying celastrol’s anti-cancer effects could be further explored through mechanistic studies. This could provide important insights into the potential molecular targets of celastrol in cancer cells and could inform the development of more targeted therapies.
Furthermore, additional clinical trials are needed to confirm the safety and efficacy of celastrol in larger patient populations with CML and other cancer types. These studies could also evaluate the long-term effects of celastrol treatment, including its impact on quality of life and survival rates. Finally, studies could investigate the potential of celastrol as a preventative agent for cancer development in high-risk populations. Celastrol’s antiinflammatory and antioxidant properties suggest that it could have a role in reducing the risk of cancer development, and future studies could investigate this potential use. Overall, the findings of our study provide a foundation for future investigations into the potential of celastrol as an anti-cancer drug and highlight the importance of natural compounds in cancer research and treatment.
References
- Morris, Christopher, Nicolas P Bebeau, Hans Brockhoff, Rahul Tandon, et al. (2015) Mandibular fractures: an analysis of the epidemiology and patterns of injury in 4,143 fractures. Journal of Oral and Maxillofacial Surgery 73( 5): 951-e1.
- Eshghpour M, Nezadi A, Moradi A, Shamsabadi RM, Rezaer N, et al. (2014) Pattern of mandibular third molar impaction: A cross‑sectional study in northeast of Iran. Nigerian journal of clinical practice 17(6): 673-677.
- Ciftci V, Guney A, Deveci C, Sanri I, Salimow F, et al. (2021) Spontaneous space closure following the extraction of the first permanent mandibular molar. Nigerian Journal of Clinical Practice 24(10): 1450-1456.
- Choudhury R, Rastogi S, Rohatgi RG, Abdulrahman BI, Dutta S, et al. (2022) Does pedicle flap design influence the postoperative sequel of lower third molar surgery and quality of life? Journal of Oral Biology and Craniofacial Research 12(5): 694-701.
- Naserzadeh, Yousef, Niloufar Mahmoudi, Elena Pakina, Imbia Anne Marie Wase, et al. (2019) Parameters Affecting the Biosynthesis of Gold Nanoparticles Using the Aquatic Extract of Scrophularia striata and their Antibacterial Properties. Journal of Nanoanalysis 2: 105-114.
- Amaral JS, Marto CM, Farias J, Pereira DA, Ermida J et al. (2022) A Pilot Randomized Controlled Clinical Trial Comparing Piezo Versus Conventional Rotary Surgery for Removal of Impacted Mandibular Third Molars. Bioengineering 9(7): 276.
- Hamed Nabahat, Melika Tahan, Faezeh AzaditalabDavoudabadi, Leonid sergeevich Kolomeitsev, Kapralova Valentina Vasilevna et al. (2021) Regeneration of Permanent Tooth Enamel (Alternative Solution of Nano-hydroxyapatite) after Exposure to Beer (Laboratory Study at the Dental Center, Moscow, Russia). Adv Dent & Oral Health 14(4): 555891.
- Shoshani-Dror D, Shilo D, Ginini JG, Emodi O, Rachmiel A (2018) Controversy regarding the need for prophylactic removal of impacted third molars: An overview. Quintessence International 49(8): 653-662.
- Terlemez A, Tassoker M, Kizilcakaya M, Gulec M (2019) Comparison of cone-beam computed tomography and panoramic radiography in the evaluation of maxillary sinus pathology related to maxillary posterior teeth: Do apical lesions increase the risk of maxillary sinus pathology? Imaging science in dentistry 49(2): 115-122.
- Faridnejad H (2022) Biostat Biom Open Access J Design and Simulation of the Source (Wiggler) and Medical Beamline of Iranian Light Source Facility (ILSF) for Medical Applications. JP Journal of Biostatistics 10(4): 555793.
- Abdilahi AM, Ashar A, Hassan J, Naqash A Comparative Study of two Flap Designs on Postoperative Sequelae after Removal of Impacted Mandibular Third Molar.
- Farhadi F, Emamverdizadeh P, Hadilou M, Jalali P (2022) Evaluation of Infection and Effective Factors in Impacted Mandibular Third Molar Surgeries: A Cross-Sectional Study. International Journal of Dentistry : 8934184.
- Domínguez M, Pina T (2020) Aging Through Cartoons: The Unbearable Lightness. Understanding the Discourse of Aging: A Multifaceted Perspective 307.
- Isah MB, Oche OM, Yunusa EU, Yunusa MA, Oladigbolu RA, et al. Perception, prevalence and correlates of depression among females attending the Gynaecological Clinic of Usmanu Danfodiyo University Teaching Hospital, Sokoto, Nigeria.
- Kang F, Sah M, Fei G (2020) Determining the risk relationship associated with inferior alveolar nerve injury following removal of mandibular third molar teeth: A systematic review. Journal of stomatology, oral and maxillofacial surgery 121(1): 63-639.
- Masinde D, Cheruiyot J. Dental Caries Experience and Associated Risk Factors Among Pre-School Going Children, In: Bureti Sub-County, Kericho County, Kenya.
- Joachim, Michael V, Yair Brosh, Camron M Rivera, Maria J Troulis, et al. (2022) Surgical Complications of Orthognathic Surgery. Applied Sciences 13(1): 478.
- Chen, Chien-Chung, Ching-Hsiang Yang, Yu-Jen Chang, Cheng-Chun Wu, et al. (2022) Repairing Facial Fractures with Interrupted Maxillary-mandibular Arches by Computer-assisted Reverse Planning Model Surgery. Plastic and Reconstructive Surgery Global Open 10(2): e4149.






























