Synthesis and Characterisation of Zinc (II) and Nickel (II) Complexes with 3-Hydroxy-4-(2-Hydroxy Phenyl) Amino Cyclobut-3-Ene-1,2-Dione
Kufre Mabel Udoisang1 and Camillus Uchenna Okonkwo2*
1Department of Chemistry, University of Uyo, Uyo City Akwaibom State, Nigeria
2Department of Environmental Engineering, College of Resources and Environment, University of Chinese Academy of Sciences, China
Submission: November16, 2022; Published: November 28, 2022
*Corresponding author: Kufre Mabel Udoisang, Department of Chemistry, University of Uyo, Uyo City Akwaibom State, Nigeria
How to cite this article: Kufre Mabel U, Camillus Uchenna O. Synthesis and Characterisation of Zinc (II) and Nickel (II) Complexes with 3-Hydroxy-4-(2- Hydroxy Phenyl) Amino Cyclobut-3-Ene-1,2-Dione. JOJ scin. 2022; 3(1): 555601. DOI: 10.19080/JOJS.2022.03.555601
Abstract
The Ligand 3-hydroxy-4-(2-hydroxy Phenyl amino cyclobut-3-ene-1,2-dione was obtained by the reaction of squaric acid and 2-aminnophenol in diethyl ether and used for the synthesis of Zinc(II) and Nickel(II) complexes. Both the ligand and the metal complexes were characterized on the basis of melting point determination, solubility test, UV-Visible Spectroscopy, and infrared spectral studies. The ligand was characterized on the basis of melting point, UV-VIS spectroscopy, and infrared spectral studies. The results obtained indicate that the ligand has a relatively high melting point of 280.2oC, π to π* and n to π* transitions attributable to the organic groups. The IR spectra showed peaks characteristics of the mixed ligand, with C=O stretch of 1643.20Cm-1 to 1680.48Cm-1 and showed peaks characteristics of the metal complexes with M-O stretch of 883.20Cm-1 to 1010Cm-1. Tentative structures are proposed for the metal complexes based on the spectral studies.
Keywords: Ligands; Complexes; UV-VIS Spectroscopy; Infrared spectroscopy
Abbreviations: VS: Very Soluble; SS: Sparingly Soluble; INS: Insoluble; S: Soluble
Introduction
The biological importance of organic ligands and metal complexes, as well as their intriguing magnetic and spectral properties, have drawn a lot of attention in the last ten years [1]. Organic ligands can be used to form compounds with metal ions [2]. One of such organic ligands which bond with metal to produce a complex is 3-hydroxy-4-(2-hydroxy phenyl) amino cyclobut-3-ene-1,2-dione (condensation reaction of squaric acid and 2-aminophenol). From a study conducted by Dharmendra and Shekar, squaric acid condensation reactions with various aliphatic diamines yielded Schiff base ligands [3]. This ligand can bond with various transition metals. Notably Zinc(II) and Nickel(II) to form a coordination compound [4]. A metal ion in solution doesn’t exist by itself; instead, it forms complex ions or coordination compounds when it combines with ligands (typically solvent molecules or sample ions) or chelating groups [5]. A complex can be created when two atoms or ions form a dative covalent bond. Typically, a metal ion and ligands precipitate in the complex formation process, pro ducing a complex with unique properties from the basic ions. The physical and chemical characteristics of the complex produced often alter. As a result of the interaction between the metal ion and the ligand, complex ion formation alters the atom’s electron configuration [5].
New qualities are characteristically produced by the new electrical configuration. Coordination compounds are another term for complexes. Coordination compounds are simply defined as having a central metal atom, typically a transition metal like zinc, nickel, copper, or iron to name a few, surrounded by a group of (2–9) additional atoms, ions, or tiny molecules known as ligands [5].The aim of this research work is to synthesize and characterize a mixed ligand of 2-amino phenol and squaric acid with Zinc(II) and Nickel(II) metal salts for the formation of a resulting metal complex. This would be achieved by:
I. synthesizing the mixed ligand 3-hydroxy-4-(2-hydroxy phenyl) aminocyclobut-3-ene-1,2-dione.
II. synthesizing Zinc (II) and Nickel(II) metal complexes with the above named ligand.
III. Characterizing the ligand and the metal complexes on the basis of UV-VIS spectroscopy and FTIR spectral studies. The present research integrates the contributions of Ajay et al [6].
Experimental
Reagents and Materials: All chemicals used in this study were obtained commercially and used without purification. Apparatus used were weighing balance, measuring cylinder, round bottom flask, beaker, capillary tube, kallen clamp variable heater, test – tube, retort stand and clamp, filter paper, reflux condenser, magnetic hot plate with magnetic stirrer, funnel, spatula, test tube and glass rod. Chemicals & Reagents used were 2-amino phenol, squaric acid, diethyl ether, methanol, ethanol, DMSO, Nickel salt, Zinc salt, distilled water, and DPPH.
Synthesis of Mixed Ligand
The ligand was prepared by dissolving 2.28g of Squaric acid in 30mL of diethyl ether and heated at the temperature of 50oC on a stirring of 350 resolutions per second, on a magnetic hot plate. After 30 minutes of heating, a light pink coloration was observed, and a clear homogeneous solution attained. 2.18g of 2-amino phenol, dissolved in 15mL diethyl ether was added drop wisely to the stirring Squaric acid solution after an hour, the magnetic stirrer was dropped as well. The resulting mixture was then allowed to cool for 20 minutes and afterwards, filtered and washed with diethyl ether repeatedly until a pure solution was observed, to remove all unreacted substances. The filtrate was covered with an aluminum foil in a beaker lightly perforated and allowed to evaporate to dryness for three days. The residue on the filter paper was dried in a desiccator over CaCl2 for 3 days. After drying a light brown precipitate was obtained. The contributions of Ajay et al. [6], Khlood et al. [7], Fugu et al. [8] and Mounika et al. [9] are all factored into the mixed ligand’s synthesis.
Proposed equation (Figure 1)
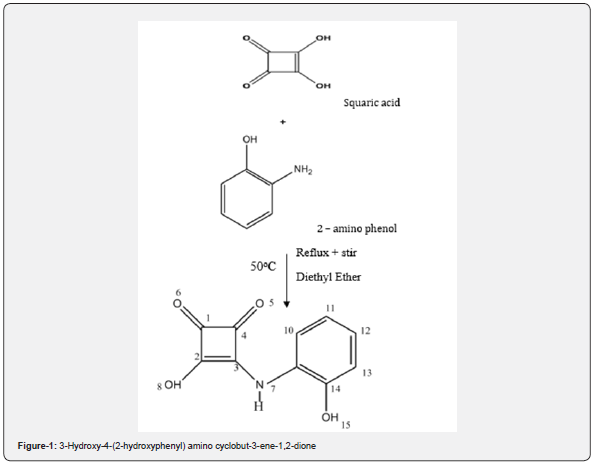
Note: Structure of squaric acid mirrored from the Journal of organic chemistry. Squaric Acid N-Hydroxylamides: Synthesis, Structure, and Properties of Vinylogous Hydroxamic Acid Analogues by Nathaniel et al [10]. Additionally, the Journal of Structural Chemistry was used to determine the structure of 2-amino phenol. Thiophenyl-2-methylidene-2-aminophenol’s crystal structure and description by Cuin et al [11].
Synthesis of Zinc metal complex of Mixed Ligand
The zinc metal complex was synthesized by dissolving 0.16g of zinc salt in 15mL methanol. 0.40g of the mixed ligand was dissolved in 30mLdiethyl ether and stirred at 350 revolutions per second at a temperature of 50oC for 30 minutes until a homogeneous solution was obtained. The dissolved zinc metal solution was added drop-wisely to the solution of the mixed ligand after which a magnet was dropped and refluxed for seven hours at a revolution of 300 revolutions per second at a temperature of 80oC. After an hour, the solution turned from brown to orange and later turned green until there was no visible reaction. The mixture was filtered, and the resulting residue was washed repeatedly with diethyl ether until a colorless filtrate was obtained and later dried in a desiccator over CaCl2 for 3 days. The filtrate was covered with a slightly perforated aluminum foil for easy evaporation of the solvent. The yield was 80.70%. The methodology employed to create the zinc metal complex was influenced by the studies of Mishra et al. [12], Shane et al. [13], Snehika et al. [14], and Pattanaik et al. [15].
Synthesis of Nickel Metal Complex of Mixed ligand.
The nickel metal complex was synthesized by dissolving 0.23g of Nickel salt in 15ml methanol. 0.40g of the mixed ligand was dissolved in 30mL diethyl ether and stirred at 350 revolutions per second at a temperature of 80oC for 30 minutes until a homogenous solution was obtained. The dissolved Nickel metal solution was added drop wisely, to the solution of the mixed ligand after which a magnetic stirrer was dropped and the mixture was refluxed for five hours with stirring, at a revolution of 300 revolutions per second at a temperature of 80oC. The mixture turned yellowish green, after an hour of refluxing, it later turned yellow and then yellowish – green after 5 hours of refluxing until there was no visible change. The resulting mixture was allowed to cool, and later filtered. The residue was washed repeatedly with diethyl ether until a colorless filtrate was obtained and later dried in a desiccator over CaCl2 for 3 days. The filtrate was covered with a slightly perforated aluminum foil, for easy evaporation of the solvent. The yield was 66%. The process employed to obtain the nickel metal complex was inspired by the research papers of Khalil et al. [16], Undegaonkar et al. [17], and Dubey et al. [18].
Melting point Determination
The melting point of the mixed ligand and that of complexes were determined using the conventional techniques of the Kallen Clanp variable heater using the capillary tube.
The melting point of the ligand and metal complexes were determined by inserting a little quantity of the sample at the sealed end of a capillary tube. The heater was adjusted to the highest heating switch and the capillary tube containing the sample was inserted in the middle hole of the variable heater. The initial temperature was observed, and the sample was viewed through a magnifying opening until a visible change was observed. The temperature at which the solid melted was read from the hold on display button of the variable heater. The experiment was carried out on the mixed ligand, the Zinc metal complex and Nickel metal complex. The method used to determine the melting point was supported by research from Dennis and Shelton [19] as well as Young and John’s observational studies [20].
Solubility Test
A solute is said to be soluble in a given solvent when a specified mixture of that solvent and a defined amount of a given solute results in a homogeneous solution [21]. The solubility of various metal complexes is characterized by using a variety of codes.
VS ………………...very soluble
SS ……………….... Sparingly soluble
INS………………... Insoluble
S …………………...soluble
About 0.005g of solid compound was added to 2mL of solvent in a test tube with vigorous shaking after each addition. If all the solute particles dissolved to give a homogenous mixture, the compound is said to be very soluble. However, if a part of the solute dissolved in the solvent, it is considered to be sparingly soluble. But if the solute remains unaffected, it is classified as insoluble.
FTIR Analysis
The sample was reduced to a fine powder and combined with a drop of liquid hydrocarbon. The combination was introduced into the IR machine between cells of potassium bromide (Kbr) and inside a smaller container. The machine was given instructions and the result of analysis was obtained. This procedure was repeated for the mixed ligand and Nickel (II) metal. In-depth understanding of FTIR analysis was provided by the works of John Coates [22], Raman et al. [23], and Maihub et al. [24].
UV-VIS Analysis
A very little portion of the sample was dissolved in DMSO, sample was premixed to desired concentrations and a blank sample of the solvent was placed on a sample holder. UV-VIS machine was turned on indicated by a green blinking light. The computer connected to the UV-VIS was logged onto the scan button was clicked on and the program was started. The wavelength ranges were changed, and the blank sample was first scanned using the baseline button. The samples were later scanned, and the data obtained was saved. Perchiammal et al. [25] and Thangadurai et al. [26] that used UV-VIS analysis as part of their research, served as an inspiration for this analysis.
Results and Discussion
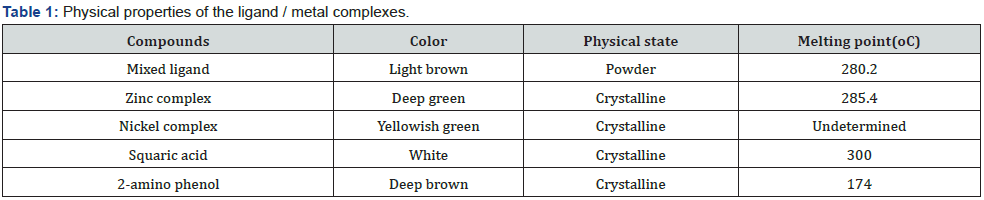
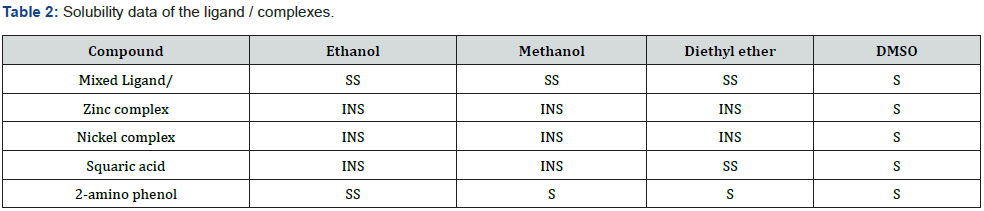
Results (Tables 1-3)
Proposed structure of the metal Complex (Figure 2)
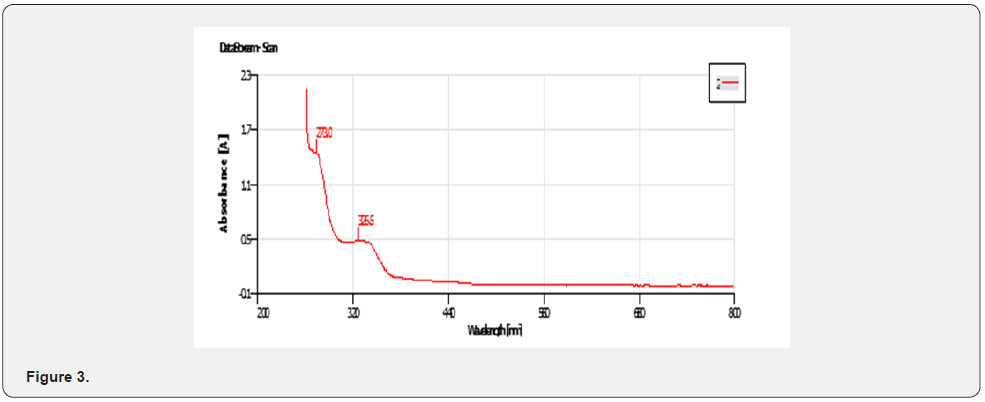
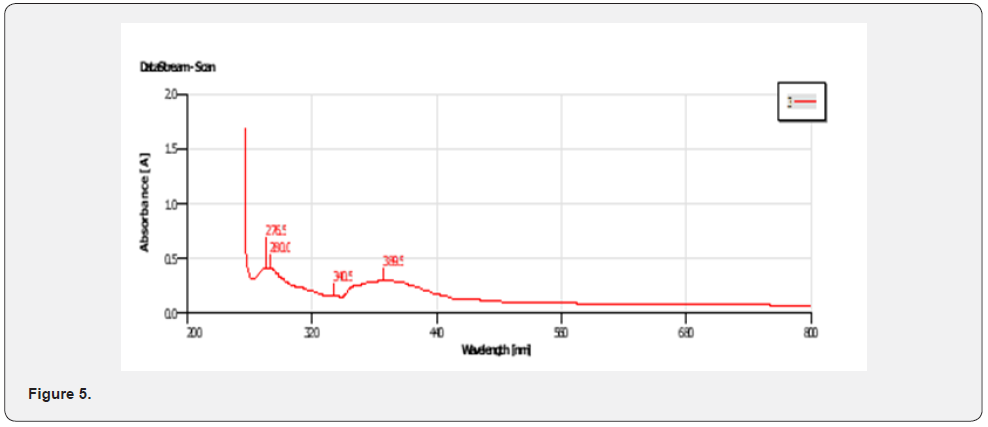
UV-VIS Peak Findings for Mixed Ligand ( Figure 3)
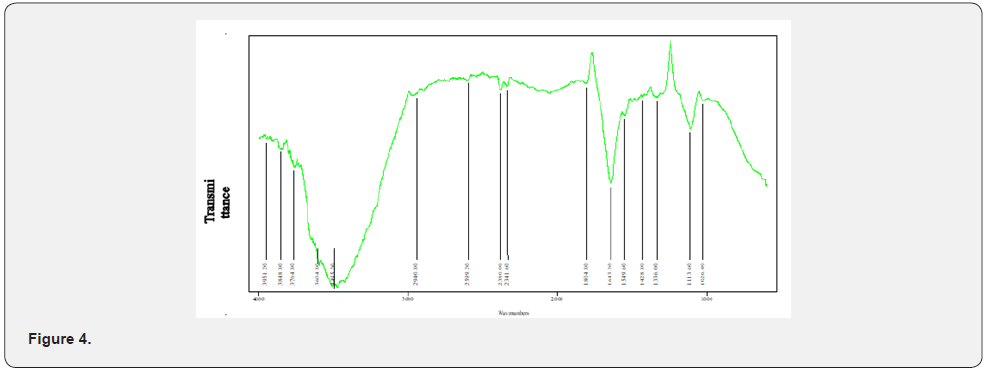
IR Peak Finding Result for Mixed Ligand( Figure 4)
Infrared Spectral Data for Mixed Ligand ( Figure 5)
UV-VIS peak finding result for Nickel Complex (Figure 5)
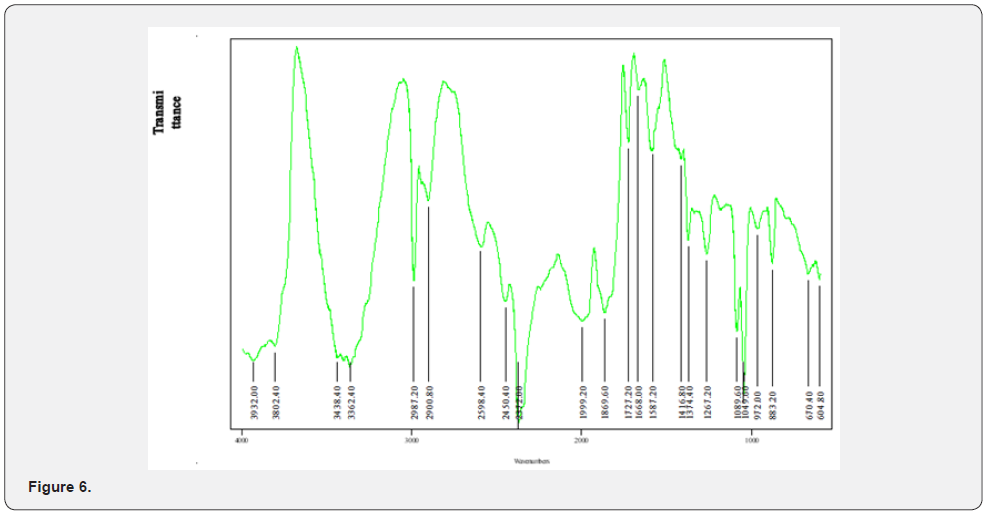
FTIR Peak Finding Result for Nickel Complex (Figure 6)
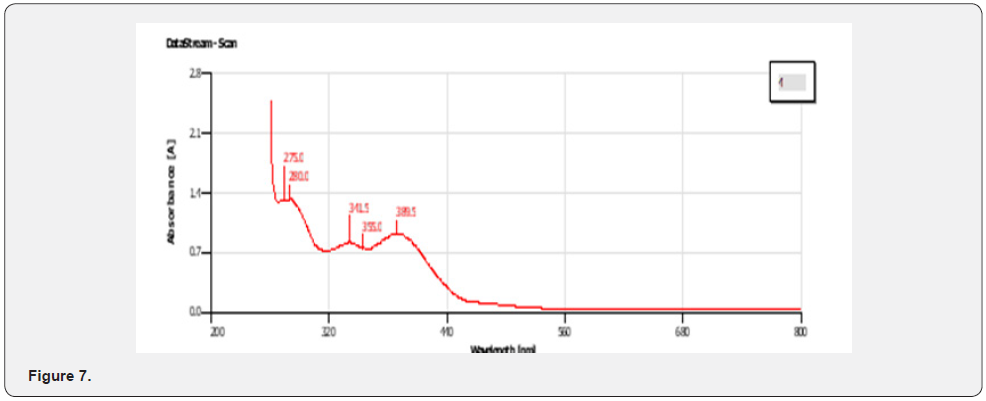
UV-VIS Peak Finding Result for Zinc Complex (Figure 7)
Metal complexes / ligand solvents


Discussion
The physical properties of the ligands and the metal complexes are presented in Tables 4.1 and 4.2. These properties include color, physical state, melting point, and solubility data. The physical state of the mixed ligand and complexes of Zinc and Nickel are crystalline. The complexes of Zinc and Nickel were found to be stable at room temperature. The melting points of the solid ranged from 200oC to 386oC indicating that there are strong interacting forces between the metals and the ligand [27]. The metal complexes were very soluble in dimethyl sulfoxide (DMSO) but insoluble in ethanol, methanol, and diethyl ether. The ligand was also very soluble in DMSO but sparingly soluble in ethanol, methanol, and diethyl other. This may be attributed to the low dielectric constant of DMSO. The UV spectra of the Mixed ligand show π to π* and n to π* transitions indicating the organic group from 2-amino phenol and there were no transitions in the visible region [28]. Two transitions occurred at 273.0nm and 326. 5nm. The UV spectra of the Nickel complex did not show any possible transition in the UV region but showed 4 transitions between 276.5nm to 389.5 nm. For the zinc complex, there are 5 transitions between 275.0nm to 389.5nm absorbance [29].
The Infrared spectra of the Mixed ligand showed peaks indicating Metal-hydrogen stretch from 2341.60cm-1 to 1840.80cm-1, H-C=O stretch at 2940.80cm-1 to 2599.20cm-1, OH stretching vibration showing a broad peak between 3200cm-1 to 3400cm-1, N-H stretch at 3495.20cm-1, C-H stretch at 2940.80cm-1 and a sharp strong peak indicating a C=O stretch between 1804cm to 1643.20cm [30].The Infrared spectra of the Nickel complex indicates Metal –Hydrogen bending vibration between 800cm-1 to 600 cm-1, Metal-Hydrogen stretching between 2230cm-1 to 1700cm-1, Metal-Nitrogen stretching vibration between 1020cm-1 to 875cm- 1 and Metal-Oxygen stretching between 1010cm-1 to 850cm-1 [31,32].
Conclusion
Zinc and Nickel complexes of 3-hydroxy-4-(2-hydroxy phenyl) amino cyclobut-3ene-1,2-dione has been synthesized and characterized based on the limited physical and spectral data, such as physical state, melting point determination, solubility test, UV-VIS spectral studies and FTIR spectral studies. There is evidence of the formation of a metal-ligand bond, and at room temperature, the solid state of both metal complexes exhibits good thermal stability.
Recommendation
I recommend that detailed structural and chemical analysis should be carried out on the metal complexes. 1HNMR, 13CNMR, x-ray diffraction technique, thermal analysis and elemental analysis should be used to confirm the tentative structures proposed for the metal complexes in view of the fact that limited data was used.
References
- More MS, Joshi PG, Mishra YK, & Khanna PK (2019) Metal complexes driven from Schiff bases and semicarbazones for biomedical and allied applications: a review. Materials today Chemistry 14: 100195.
- Bisikalo AL, Aprelkova NF (2015) Synthesis, composition, and properties of metal complexes with organic ligands derived from glucose. Russian Journal of Applied Chemistry 88: 1334–1337.
- Dharmendra KS, Shekar S (2021) Synthesis and characterization of Cr (III)Macrocyclic Schiff Base Complexes. Asian Journal of Research Chemistry14(4): 269-400.
- Saiyed T, Adeyemi J, Onwudiwe D(2021) The structural chemistry of Zinc (II) and Nickel (II) Di thiocarbamate complexes. Open Chemistry 19(1): 974-986.
- Haas KL, Franz KJ (2009) Application of Coordination Chemistry to explore and manipulate cell biology. Chem Rev 109(10): 4921-4960.
- Ajay RP, Kamini JD, Sambhaji SR, Vishwanath RP, & Rama SL (2012) Synthesis, characterization and biological activity of mixed ligand Co(II) complexes of schiff base 2-amino-4-nitrophenol-n-salicylidene with some amino acid. Journal of Chemical and Pharmaceutical Research 4(2): 1413-1425.
- Khlood S. Abou-Melha (2008) Octahedral Co(II) and Ni(II) Complexes of Schiff bases, semi carbazone and thio semi carbazone, synthesis, biological, and thermal studies. Journal of Coordination Chemistry, 61(13): 2053-2067.
- Fugu MB, Ndahi NP, Paul BB, Mustapha AN (2013) Synthesis, Characterization, and Antimicrobial Studies of Some Vanillin Schiff Base Metal (II) Complexes. Journal of Chemical and Pharmaceutical research 5(4): 22-28.
- Mounika K, Anupama B, Pragathi J, Gyanakumari C (2010) Synthesis Characterization and Biological Activity of a Schiff Base Derived from 3-Ethoxy Salicylaldehyde and 2-Amino Benzoic acid and its Transition Metal Complexes. Journal of Scientometric Research 2(3): 513-524.
- Nathaniel CL, Martha DM, Hillary AJ, Christian B (2003) Squaric Acid N-Hydroxylamides: Synthesis, Structure, and Properties of Vinylogous Hydroxamic Acid Analogues. J org Chem 68(24): 9233-9241.
- Cuin A, Pereira GA, Bortoluzzi AJ et al. (2013) Characterization, and crystal structure of thiophenyl-2-methylidene-2-aminophenol. Journal Structural Chem 54: 269–273.
- Mishra A, Purwar H, Jain R, Gupta S (2012) Microwave Synthesis, Spectral, Thermal and Antimicrobial Studies of Some Co(II), Ni(II)and Cu(II) Complexes Containing 2-Aminothiazole Moiety. E-Journal of Chemistry 9(4): 1655-1666.
- Shane M, Timothy M, Elizabeth J (2016). Synthesis and Characterization of Metal Complexes with Schiff Base Ligands. Journal of Chemical Education. 93(2): 351-354.
- Snehika S, Kumar A, Dikshit SN (2009) Synthesis, Spectral and Biological Studies of Cu(II), Zn(II) and Cd(II) Complexes with Schiff Base Ligand. Asian Journal of Chemistry 21(9): 7224-7228.
- Pattanaik S, Rout S, Panda J, Sahu P, Banerjee M (2011) Synthesis, characterization, and biological evaluation of bidentate ligands (Reduced Schiff's Base) with metals of copper, nickel, and zinc complexes. Rasayan J Chem 4(1): 136-141.
- Khalil A, Sinann A, Bayati I, Anaam R (2016) Synthesis, Characterization, Thermal Study and Biological Evaluation of Transition Metal Complexes Supported by ONNNO – Pentadentate Schiff Base Ligand. American Journal of Chemistry6(1):1-7.
- Undegaonkar MG, Sinkar SN, Bhosale V N, Mirgane SR (2021) Solvent free synthesis and characterization of few metal complexes of Schiff base derived from 2-Amino-5, 6-dimethyl benzimidazole & Syringaldehyde. International Journal of Pharmaceutical Sciences and Drug Research13(3): 240-245.
- Dubey R, Dubey U, Mishra C (2008) Synthesis and physicochemical characterization of some Schiff base complexes of chromium (III). Indian Journal of Chemistry. 47(A): 1208-1212.
- Dennis, L M, Shelton RS (1930) An apparatus for the determination of melting points. Journal of the American Chemical Society 52(8): 3128-3132.
- Young J, John CO (2013) True Melting Point Determination. Chem Educator 18: 203-208.
- Muammer C. Alipasa A (2005) A cross-age study on the understanding of chemical solutions and their components. International Education Journal. 6(1): 30-41.
- John coates(2006) Interpretation of infrared spectra, a practical approach. Encyclopedia of analytical chemistry. pp. 10815-10837.
- Raman N, Daveethu JR, Sakthivel A (2007) Synthesis, Spectral Characterization of Schiff Base Transition Metal Complexes: DNA Cleavage and Antimicrobial Activity Studies. Journal of Chemical Sciences 119(4): 303-310.
- Maihub A, Marei E, Salah B, Naghmush A (2006) Template synthesis and spectroscopic characterization of some Schiff base complexes of transition metal ions. Asian Journal of Chemistry 18(4): 2431-2436.
- Pechiammal M, Ravichandran S, Sing L (2014) Synthesis, characterization and antibacterial activity of metal conplexes from mannich base, N-[1- morpholidino(4-nitrobenzyl)] acetamide. International Journal of Chem Tech Research 6(7): 3680-3684.
- Thangadurai T D, Ihm SK (2003) Chiral Schiff base ruthenium (III) complexes: Synthesis, characterization, catalytic and antibacterial studies. Journal of Industrial and Engineering Chemistry 9(5): 563-568.
- Taleb TA (2009) Synthesis and characterization of metal complexes of Cr(III), Mn(II), Fe(II), Co(III), Ni(II), Cu(II), Ru(II), and Pd(II) with derivatives of 1,3,4-thiadiazole-2,5-dithiol as new ligands. Journal of saudi chemical society 13(3): 253-257.
- Ade S, Kolhatkar DG, Deshpande MN (2012) Synthesis, characterization and biological activity of a schiff base derived from isatin and 2-amino, 4-methyl phenol and its transition metal complexes. International Journal of Pharma and Bio Sciences 3(2): 350-356
- Surech MS, Prekash V (2010) Preparation and Characterization of Cu(II), Mn(II), Co(III), Ni(II), Co(II) and Cd(II) Chelates of Schiff Base Derived from Vanillin and 4-aminoantipyrine. International Journal of physical science 5(14): 2203 -2211.
- Mapari AK ,Mangaonkar KV (2011) Stability Constant of Mixed Ligand Complexes of Transition Metal (II) Ions with N-(2-hydroxybenzyldene)-2,3-dimethylaniline as Secondary Ligand. E-Journal of chemistry 1(8): 123-126.
- Samanta B, Chakraborty J, Shit S, Batten S, Jensen P, et al. (2007) Synthesis, characterization and crystal structures of a few coordination complexes of nickel(II), cobalt(III) and zinc(II) with N′-[(2-pyridyl) methylene] salicyloylhydrazone Schiff base. Inorganica Chimica Acta 360(7): 2471-2484.
- Singh N, Kushawaha S, Dixit AK (2000) Synthesis, characterization and biological activity of the complexes of manganese(II), iron(II), cobalt(II), nickel(II), copper(II), zinc(II) and cadmium(II) with N-Benzoyl-N′-2-furanthiocarbohydrazide. Indian Journal of Chemistry 39(10): 1070-1073.






