Effects of Graded Quantities of Xanthan Gum on the Physicochemical and Flocculation Properties of Gum Arabic
Ezera Joshua E, Nwufo Berthrand T and Wapwera Jidimma A*
Department of Chemistry, University of Jos, Nigeria
Submission: January 31, 2019; Published: March 18, 2019
*Corresponding author: Wapwera Jidimma A, Department of Chemistry, University of Jos, Nigeria
How to cite this article: Ezera J E, Nwufo BT, Wapwera J A*. Effects of Graded Quantities of Xanthan Gum on the Physicochemical and Flocculation Properties of Gum Arabic. JOJ scin. 2019; 2(1): 555579. DOI: 10.19080/JOJS.2018.01.555579
Abstract
The physicochemical properties of graded quantities Gum Arabic and Xanthan gum were determined and compared with its individual properties. For its application in Food and pharmaceutical purposes, Gum Arabic aqueous solutions were modified using different graded quantities of Xanthan gum and tested for their Physico-Chemical and Flocculation properties using standard procedures and different laboratory equipments. It was observed that Xanthan gum has a high viscosity property at a low concentration and improved the Physico-Chemical and Flocculation properties of the Gum Arabic. As the quantities of Xanthan gum were added into the Gum Arabic in an increasing proportion, properties such as viscosity, specific gravity and pH were generally improved. From the results obtained, Xanthan can be used as an alternative means for other materials used alongside Gum Arabic for the formulation of additives as emulsifiers and thickeners in food and pharmaceutical industries.
Keywords: Gum Arabic; Xanthan gum; Food and pharmaceutical purposes; Xanthan; Emulsifiers; Additives; Food and pharmaceutical industries; Flocculation properties; laboratory equipments; Insoluble pharmaceuticals
Introduction
Gum is an adhesive substance of vegetable origin, mostly obtained as exudates from bark of trees Nishinari K [1]. Gums are generally formed from the disintegration of internal plant tissues, mostly from decomposition of cellulose in a process known as gummosis. Gums contain high amounts of polysaccharides of natural origin and are closely allied to pectin. They are colloidal and soluble in water either dissolving entirely or swelling, but they are insoluble pharmaceuticals, and food.
Gums are produced by making incision in the bark of the tree and collecting exudates repeatedly throughout the season. Trees produce gums by a process called gummosis, possibly a protective mechanism, either after mechanical damage to the bark or after bacterial, insect or fungal attack upon it. Gums whether natural, synthetic or semi-synthetic can be classified into two groups namely acid gums and neutral gums. Acid gums include glucuronic acid, galacturonic acid, sulphate group and phosphate group. The neutral gum includes the hexose and pentose. Gums can also be classified as hydrophilic or hydrophobic Mhinzi GS [2].
Natural gums occur in all life forms. We use gum Arabic for sealing envelopes and for sticking postal stamps. The better consistency to ice-cream is often provided by gelatin or dextran. The delicious jellies and jams can be moulded into beautiful shapes using China grass (sea weed, agar). Gum tragacanthin gives desirable flow property to the toothpaste (Figure 1).
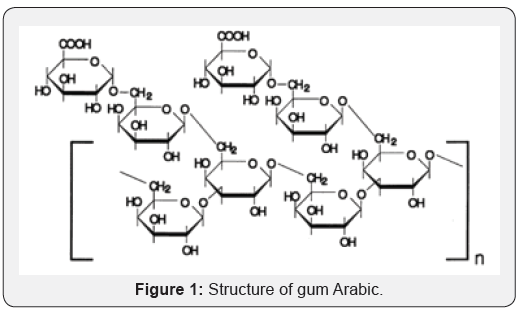
According to Onoja E [3], it has been revealed that gum Arabic has a wide range of industrial usage. The various industries where gum Arabic has been used extensively as binder (film former) include; food industry, pharmaceutical industry, medicine, cosmetic industry, paint industry and several other manufacturing industries.
In paints formulation, gum Arabic is used as binder where it prevents hard setting of pigments. Such controlled flocculation has frequently been achieved by means of additives which presumably affects the coherence of particles in immiscible liquids Glicksman M [4].
Xanthan gum
Xanthan gum was discovered in the 1950s by Dr Allene Rosalide Jeanes, a chemist working with the Northern Regional Research Laboratory of the U.S. Department of Agriculture in the course of a screening which aimed at identifying microorganisms that produced water-soluble gums of commercial interest. The first industrial production of xanthan was carried out in 1960, and the product first became available commercially in 1964. Other names for xanthan gum are Corn sugar gum Toxicology and safety studies showed that xanthan caused no acute toxicity, had no growth-inhibiting activity, and did not alter organ weights, hematological values or tumors when fed to rats or dogs, neither in short-term, nor in long-term feeding studies.
The approval for food use was given by the U.S. Food and Drug administration in 1969, and the FAO/OMS specification followed in 1974. Authorization in France was given in March 1978, and approval in Europe was obtained in 1982, under the E number E415. The official definition of the EU food regulations for E415 is: Xanthan gum is a high molecular weight polysaccharide gum produced by a pure culture fermentation of a carbohydrate with natural strains of Xanthomonascampestris, purified by recovery with ethanol or propane-2-ol, dried and milled.
It is then added to a liquid medium to form a gum. It contains d- glucose and d-mannose as the dominant hexose units, along with d-glucuronic acid and pyruvic acid, and is prepared as the sodium, potassium or calcium salt. Its solutions are neutral. Xanthan gum is approved as food additive with an acceptable daily intake (ADI) not specified; that is, no limit for ADI is defined and the gum may be used quantum satis, which means with just the quantity useful for the application. Today, xanthan is produced commercially by several companies, such as Monsanto/Kelco, Rho-dia, Jungbunzlauer, Archer Daniels Mid-land, and SKW Biosystems.
Structure of xanthan
Xanthan is a natural polysaccharide and an important industrial biopolymer. It is naturally produced by the fermentation with the bacteria Xanthomonascampestries. Its properties allow it to supplement other known natural and synthetic water-soluble gum. Each xanthan gum repeat unit, shown in Figure 2, consists of five sugar residues: two glucose, two mannose, and one glucuronic acid. The polymer backbone consists of 1, 4-linked ß-D-glucose and is therefore identical in structure to cellulose. Each side chain comprises a glucuronic acid residue between two mannose units. At most of the terminal mannose unit is a 4, 6-linked pyruvate acid; the mannose nearest the main chain typically carries a 6-linked acetyl ester or can also be unsubstituted (Figure 2).
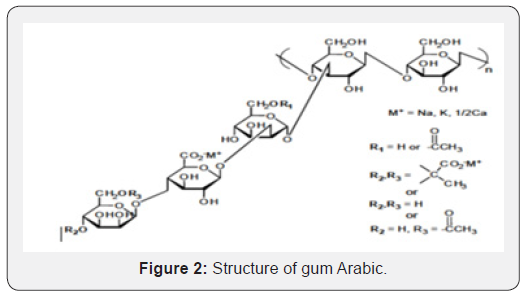
Application of xanthan gum
Food application: In foods, xanthan gum provides stability and improves or modifies textural qualities, pouring characteristics and cling. In beverages, a slight increase in viscosity imparts the sensation of enhanced body without reducing flavor impact. Partially replacing high concentrations of starch in many food systems with xanthan gum contributes to a more pseudoplastic rheology. The benefits are improved flavor release and more pleasing texture.
Beverages: Xanthan gum in dry-mix beverage bases provides enhanced body and quality to the reconstituted drink, along with rapid viscosity development. In addition, it uniformly suspends fruit pulp in prepared drinks to improve product appearance and texture.
Confectionery: Marshmallow toppings containing xanthan gum retain uniform air-cell structure through-out the products’ shelf life. Xanthan gum/locust bean gum blends improve manufacturing efficiency of starch jelly candies by accelerating setting time.
Dairy products: Blends of xanthan gum, carrageenan, and galactomannans are excellent stabilizers for a range of frozen and chilled dairy products: ice cream, sherbet, sour cream, sterile whipping cream and recombined milk. This economical blend provides quality texture and improves its shelf-life.
Aim
This study is aimed at using Xanthan gum for modification of Gum Arabic to improve its physicochemical properties for use in Food and Pharmaceutical industries.
Objectives of the Study
a) The objectives of this work are to:
b) To determine the physicochemical properties of Gum Arabic and Xanthan Gum
c) To prepare gum solutions of graded mixtures of Gum Arabic and Xanthan Gum
d) To investigate the effect of the addition of Xanthan gum to gum Arabic viscosity.
e) To study the viscosity behavior of the graded gum samples in order to improve gum quality
Materials and Method
Sample collection and description
The Gum arabic samples were obtained from three different Acacia tree gum exudates found in the market place of Jos North LGA, Plateau State. Samples were collected as dry nodules or lumps. The method of collection was hand-picking selection of gum (HPSG), this ensured picking white clean nodules which are the most expensive grades of the exudates. The gum Samples were then kept in separate (labelled) bags. The Xanthan gum samples were collected from Nasco Household company Jos, Plateau state. The two samples were collected in an air tight labeled bags finely processed and stored for analysis.
Preparation of samples
The gum samples consisted of mixtures of large and small nodules admixed with bark and organic debris. Hand-picked select gum (HPSG) method was used to separate the neat, quality gum from other constituents. The former was then spread out under room shade until dry. The dried samples (hard nodules) were then ground into fine powder (to pass 0.4mm mesh screen). The prepared samples were kept in tight containers and stored at room temperature until required for subsequent analysis.
Experimental methods
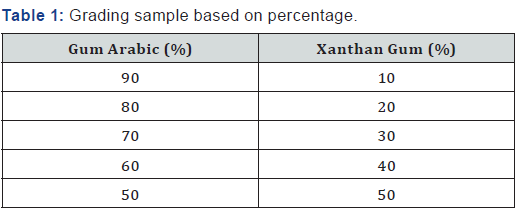
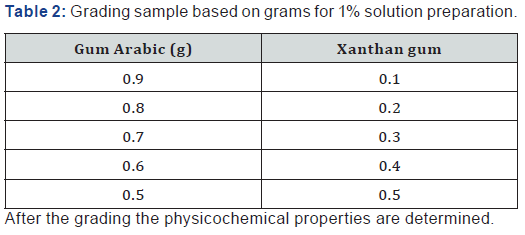
Physicochemical analysis of samples under study was done within the departmental laboratory. Each analysis was repeated three times, and values reported in respect of the gum samples are actually the average of three replications (Table 1-7).
Physical characteristics of the gums
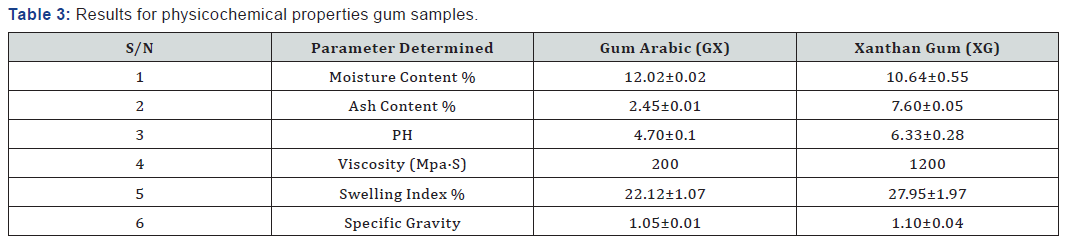
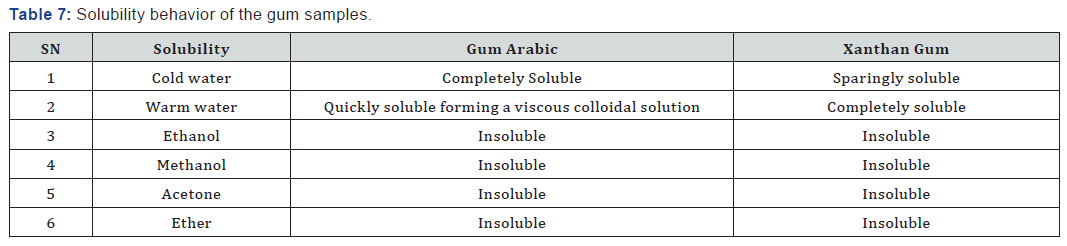
Both the Gum Arabic and Xanthan Gum samples were found to be water soluble at 300C to form viscous solutions, indicating that they are natural gums of the hydrophilic colloid group. Samples were however found to be insoluble in common organic solvents (ethanol, acetone, ether, chloroform, benzene etc.) and in oils, with which they form emulsions in aqueous suspension. The good solubility of these gums is also indicative of the absence of cross linking between polymeric chains. This is because gums having cross linked polymeric chains only swell in water, without dissolving.
Gum solution density increased with increasing solution concentration, with Gum Arabic having higher solution density than Xanthan Gum. Density is a measure of the degree of compact packing of macromolecules in the gums. The following physicochemical characteristics were carried out on the graded gum samples; moisture content, ash content, pH, viscosity, swelling index, specific gravity.
Moisture content and ash content
The moisture content and ash content were determined according to AOCA [5] standard. From the experimental result in Table 1, the moisture content of gum Arabic was higher than Xanthan gum. The moisture content value for gum Arabic was within the range ≤15 which is according to international specification. Also, the moisture content of Xanthan gum was within the range of 8-15 according to international specification.
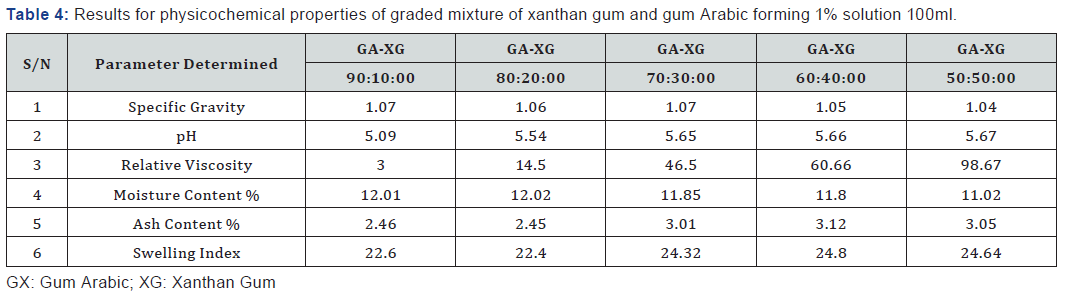
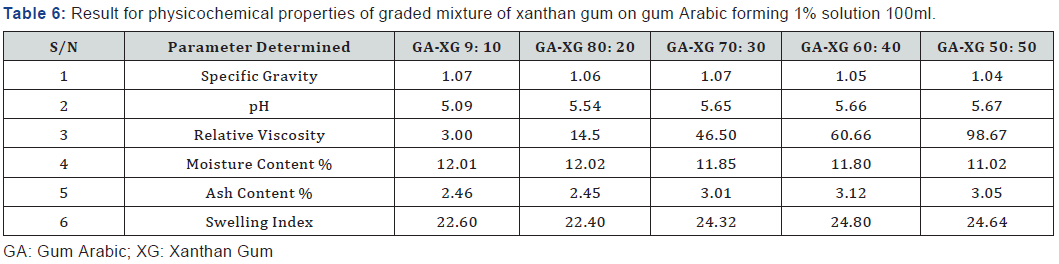
The graded mixture of Xanthan gum to gum Arabic reduced the moisture content of the gum Arabic as seen in Table 2 even as the quantity of xanthan was increased in varying ratios. This observation suggests that the graded mixture of gum Arabic (GA) and Xanthan Gum (XG) if used in formulation improves the quality of the gum when applied in food industries and beverages to prevent caking of beverages due to high moisture content and increase the shelf life. According to Shittu AS, Mahmuda moisture content of 12.02% of gum Arabic at 300C is very high and as such moisture sensitive drugs should be packaged in well protected packages to prevent spoilage. The ash content of Gum Arabic is 2.45% while the Xanthan gum is 7.60%. The very high volume of ash content shows that Xanthan gum has higher mineral content compared to gum Arabic, which indicates that Xanthan Gum has higher mineral content compared to gum Arabic.
pH properties
The pH of the aqueous solution of the gum Arabic indicated an acidity of 4.70 while the pH of Xanthan was slightly acidic with a value of 6.33. The graded mixture of quantities of Xanthan gum on Gum Arabic altered the pH value of the gum Arabic. As the ratio of the Xanthan gum was increased in a 1% solution of the graded mixtures, the pH of the graded quantities also increased. From table 2, it is observed that the pH value of the 90:10 grade of the 1% solution is in the range of 4-5 which is of specification given by FAO, for gum Arabic. According to Anderson [6]; the increase in pH of gum Arabic results in maximum viscosity around pH 5.0-5.5 for a gum Arabic solution.
Viscosity
Gum Arabic has high water solubility and relatively low viscosity compared with Xanthan gum. The relative viscosity of gum Arabic increased as the ratio of Xanthan gum added to the solution was increased from Table 2. The relative viscosity of 1% solution was very low showing that the concentration of the gum Arabic is very low. When graded quantities of Xanthan gum were added to gum Arabic to form a 1% solution, a viscous solution was formed. The viscosity increased as the ratio of the added quantities of Xanthan gum increased.
According to Williams, Solutions containing less than 10% of gum Arabic have a low viscosity and responds to Newtonian behavior while solutions of 40% of gum Arabic responds to pseudoplastic behavior. Also, it is seen that even 30% gum Arabic solutions have a lower viscosity than 1% xanthan gum at low shear rates. In addition, while gum Arabic is Newtonian in behavior with viscosity being shear rate independent, xanthan gum display non-Newtonian shear thinning characteristics, Therefore addition of little quantity of xanthan gum highly increased the viscosity of a gum Arabic solution and causes it to have a pseudoplastic and synergistic nature which improves its properties especially in the food and beverage industries and also in paint industries. This ensures excellent flow control even at low concentrations.
Emulsifying properties (flocculation)
Emulsions are from physicochemical point of view, thermodynamically unstable systems which rapidly or slowly separate into two immiscible phases according to the kinetic stability. According to Comas et al. [7], mechanisms of physical destabilization of emulsions include oil droplet size variation process such as flocculation, coalescence and particle migration. Microbial and plant gums as well as some plant and animal proteins have been known to possess lipid emulsifying effects. Especially, xanthan gum with microorganism origin has been widely used in the food industry because of its high emulsifying activity Cirigliano & Carman [8].
When both gum Arabic and Xanthan gums are mixed, the resulting mixtures exhibited a translucent homogeneous aspect, with no phase separation visible to the naked eye which indicates that the mixture as synergistic. The droplet size distribution was homogenous when the rate of Xanthan gum added was low. As the grade of Xanthan gum was increased, its homogeneity decreased.
There was no visible flocculation or sedimentation of the graded quantities of the gum solutions during extended storage (7 days). The xanthan-gum Arabic mixture blocks inhibit flocculation and coalescence through electrostatic and steric repulsions Williams PA [9]. This indicates that the Gum Arabic- Xanthan Gum conjugates could produce efficiently stable emulsions and inhibit flocculation of the aqueous mixture Dickson [10]. Therefore, it confers on gum Arabic solution a unique property of long-lasting suspension of particulates, even in complex formulations.
Swelling index
Swelling index is the water holding capacity of the gum samples and the capacity at which they attain swelling equilibrium. Xanthan gum has a higher swelling index 27.95% compared to gum Arabic which had a lower swelling index of 22.12%, this signifies the hydrophilic nature of gum. This implies that the gum has the capacity to swell into a gelatinous material from which any embedded drug could be released. The mixture of graded quantities of Xanthan gum to Gum Arabic increases the swelling index of the gum solution due to the ability of xanthan gum to hold water and swell. This improves the binding capacity of gum Arabic for usage in pharmaceutical industries as tablet binders [11-53].
Conclusion
The study showed that there is a special relationship between gum Arabic and xanthan gum. The isometric properties of xanthan-acacia gum aqueous mixtures have been characterized and viscosity rise was observed when the xanthan gum was added to the solutions of gum Arabic, which clearly shows that xanthan and acacia molecules do interact, thus resulting in a so-called synergistic mechanism. Also, the isometric results clearly indicate that the extent of interactions between both gums is governed by their structural and chemical characteristics. The present study clearly demonstrated that the graded mixture Xanthan gum and gum Arabic at certain concentration could significantly improve the solubility and emulsify¬ing properties of gum Arabic. Also, the graded sample could improve the shelf-life of food products if used in formulation due to mineral contents determined from aching of gum samples.
The main contribution to the improved long-term stability of gum Arabic is Xanthan gum in the conjugate providing a bulkier steric stabilizing layer in the droplets to have maximum emulsion stability.
Recommendation
Xanthan gum (GX) and acacia gum (GA) graded mixtures should be employed in food industry, indeed xanthan gum is used for its thickening properties of aqueous solutions and acacia gum for its emulsifying ability. The present work studied the effect of mixtures on the physicochemical properties of the gum Arabic. More research should be carried out on the impact of the graded mixture on the chemical structure of each gum, determination of mineral content of the graded quantities and Atomic Absorption Spectroscopy should also be carried out on the graded samples to quantitatively determine the minerals present. This would help discover mixture formulae that would improve the emulsifying and stabilizing properties in food industries and pharmaceutical industries.
References
- Nishinari K, Nitta Y, Kim BS (2003) Journal Food Science 23: 936.
- Mhiniz GS (2003) Sci Fo Agric 83: 142.
- Onoja E (1998) The film forming ability of gum Arabic. project, University of Jos, Nigeria.
- Glicksman M (1973) Gum arabic, industrial gums, polysaccharides and their derivatives. Academic press, pp. 234-236.
- (1990) Association of Official Analytical Chemists. Official Methods of Analysis, (15th), Washington DC, USA.
- Anderson DMW, Brown Douglas DM, Morrison NA, Weiping W (1990) Specifications for gum arabic (Acacia Senegal); analytical data for samples collected between 1904 and 1989. Food Addit Contam 7(3): 303-321.
- Cosmas (2006) Effects of parenteral Infusion with sunflower-oil emulsion. Applied veterinary Clinical Nutrition.
- Cirigliano MC, Carman GM (1985) Purification and characterization of liposan, a bioesmulsifier from Candida lipolytica. Appl Environ Microbiol 50(4): 846-850.
- Williams PA, Phillip GO (1998) Gums and Stabilisers for the Food Industry 9 Royal Society of Chemistry, Cambridge UK.
- Dickinson E (1992) An introduction to food colloids. 207 Seiten, zahlr. Abb. Oxford University Press, Oxford, New York, USA.
- Abu BA, Tahir A, Sabah E (2007) Effect of Tree and Nodule Age on some Physicochemical Properties of Gum from Acacia Senegal (L.) Willd Sudan. Research Journal of Agriculture and Biological Sciences 3(6): 866-870.
- Ahmed SE, Mohamed BE, Karamalla KA (2009) Analytical Studies on the Gum Exudates from Anogeissus leiocarpus. Pakistan Journal of Nutrition 8(6): 782-786.
- Al-Assaf, Phillips S, Sasaki Y (2006) Characterization and properties of A. Senegal Willd. Var. Senegal with enhanced properties”. Part 1: Controlled maturation of A. Senegal var. Senegal to increase viscoelasticity, produce hydrogel form and convert a poor into a good emulsifier, Food Hydrocolloids.
- Mahmoudi AS (2010) Pharmacological Research 62: 144-149.
- Baker CW, Whistler RL (1975) Distribution of D galactosyl groups in guar and locust bean gum. Carbohydrate Research 45: 237-243.
- Bayerlein F, Kuhn M, Maton M (1984) South African patent ZA 84-07492. Biotechnology Progress 6: 182-187.
- Carter SJ (2005) Tutorial Pharmacy, Pitman Press, Great Britain.
- Chikamai BN, Mugah JO, Chikamai BN, Mbiru SS, Casadei E (1997) Production, markets and quality control of gum Arabic in Africa: Findings and recommendations from an FAO project.
- Clark PE, Halvaci M, Ghaeli H, Parks CF (1985) Proppant transport by xanthan and xanthan hydroxypropyl guar solutions”: alternatives to cross-linked fluids. In Society of Petroleum Engineers, SPE paper #13907, Denver, Colorado, USA, pp. 577-582.
- Clark RC (1987) Viscoelastic response of xanthan gum/guar gum blends. IRL Press 4: 165-172.
- Clark RC, Sanderson GR (1984) For a bacterial polysaccharide in solution: a role for polysaccharide conformation in recognition between Xanthomonas pathogen and its plant host. J Mol Biol 110: 1-16.
- Cuvelier, Launay B (1986) Concentration regimes in xanthan gum solutions deduced from flow and viscoelastic properties. Carbohydrate Polymers 6(5): 321-333.
- Dickinson E (2003) Hydrocolloids at interfaces and the influence on the properties of dispersed systems. Food Hydrocolloids 17(1): 25-39.
- Dickinson E (2009) Hydrocolloids as emulsifiers and emulsion stabilizers. Food Hydrocolloids 23(6): 1473-1482.
- Dickinson E, Elverson DJ, Murray BS (1989) On the film-forming and emulsion-stabilizing properties of gum Arabic: dilution and floculation aspects. Food Hydrocolloids 3(2): 101-114.
- Drakos A, Kiosseoglou (2008) Depletion flocculation effects in egg-based model salad dressing emulsions. Food Hydrocolloids 22(2): 218-224.
- Garcõ Âa-Ochoa F (2000) Biotechnology Advances.
- Flores Candia JL, Decker WD (1999) Effect of the nitrogen source on pyruvate content and rheological properties of xanthan. Biotechnol Prog 15(3): 531-538.
- García Ochoa F, Santos VE, Casas JA, Gomez E (2000) Xanthan gum: production, recovery, and properties. Biotechnol Adv 18(7): 549-579.
- Garti I, Leser M (2001) Emulsification properties of hydrocolloids. Polymers for Advanced Technologies 12(1-2): 123-135.
- Glicksman M (1969) Gum Technology in the Food Industry. Academic Press, New York, USA.
- Glicksman M (1982, 1983, 1986) Food Hydrocolloids. (Volume I,II,III), CRC Press, Boca Raton, Florida, USA.
- Hassler RA, Doherty DH (1990) Genetic engineering of polysaccharide structure: production of variants of xanthan gum in Xanthomonas campestris. Biotechnol Prog 6(3): 182-187.
- Hemar Y, Tamehana M, Munro PA, Singh H (2001) Influence of xanthan gum on the formation and stability of sodium caseinate oil in water emulsion. Food Hydrocolloids 15(4-6): 513-519.
- Jeanes A, Pittsley JE, Senti FR (1961) Introduction to Xanthan-References.
- Hunter RJ (1986) Foundations of colloid science. (Volume 1), Oxford: Oxford Science.
- Idris OHM, Williams PA, Phillips GO (1998) Characterisation of gum from Acacia Senegal trees of different age and location using multidetection gel permeation chromatography. Food Hydrocolloids 12(4): 379-388.
- Imeson A (1992) Thickening and Gelling Agents for Food Blackie Academic and Professional Publishers, Glasgow, UK.
- Karamalla KA, Siddig NE, Osman ME (1998) Analytical data for Acacia Senegal gum samples collected between 1993 and 1995 from Marline: Optimizing the adhesive property of plant exudates (gum Arabic). pp. 13-15.
- Morris EW, Darke A (1977) Order-disorder transition.
- Nussinovitch A (1997) Hydrocolloid Applications; gum technology in the food and other industries. Blackie Academic and Professional, London.
- Phillips GO, Williams PA (2001) Tree exudate gums: natural and versatile food additives and ingredients. Food Ingred Anal Int 23: 26.
- Randall R C, Phillips GO, Williams PA (1998) Food Hydrocolloids 2: 131-132.
- Fenyo JC, Vandervelde MC (1990) In Gum and Stabilizer for the Food Industry. IRL Press: Oxford 5: 17-23.
- Seigler DS (2002) Phytochemistry of Acacia-sensulato. Biochemical Systematics and Ecology 31(8): 845-873.
- Baldwin TC, Quah PE, Menzies AR (1999) A serotaxonomic study of Acacia gum exudates. Phytochemistry 50(4): 599-606.
- Anderson DM, Douglas DM, Morrison NA, Wang WP (1990) Specifications for gum Arabic (Acacia Senegal); analytical data for samples collected between 1904 and 1989. Food Addit Contam 7(3): 303-321.
- Stephen AM (1995) Food Polysaccharides Marcel Dekker, New York, USA.
- Li X, Fang Y, Al-Assaf S, Phillips GO, Nishinari K (2009) Rheological study of gum Arabic solutions: Interpretation based on molecular self-association. Food Hydrocolloids 23(8): 2394-2402.
- Talashek T, Seheult M, Carter T, Navarrete R, Chang H (2009) Xanthan Biosynthesis by Xanthomonas Bacteria: An overview of the current Biochemical and genomic data.
- Verbeken S, Dewettinck (2003) Exudate gums: Occurrence, production, and applications. Appl Microbiol Biotechnol 63(1): 10-21.
- Whistler RL, Bemiller JN (1993) Industrial Gums; polysaccharides and their derivatives. (3rd), Academic Press, San Diego, USA.
- Whistler RL (1973) Industrial Gum Academic Press, New York, USA, p. 7-8.






























