Investigation of Interactional Behavior of some Biologically Important Compounds by a Quantitative Method
Khadem Sadigh Mahsa* and Zakerhamidi MS
Research Institute for Applied Physics and Astronomy, University of Tabriz, Iran
Submission: July 11, 2018; Published: August 02, 2018
*Corresponding author: Mahsa Khadem Sadigh, Research Institute for Applied Physics and Astronomy, University of Tabriz, Iran; Tel: +98-41-33393027; Fax: +98-41-33347050; Email: mahsa.sadigh@tabrizu.ac.ir
How to cite this article: M. Khadem Sadigh, M.S. Zakerhamidi. Investigation of Interactional Behavior of some Biologically Important Compounds by a Quantitative Methods. JOJ scin. 2018; 1(4): 555568. DOI: 10.19080/JOJS.2018.01.555568
Abstract
The features of surrounding environment of biologically interesting compounds can play key role in their function in various phenomena. Environment effects are usually studied qualitatively, but this method cannot give precise information about the interactional behavior of under study molecules. In this case, a quantitative interpretation can give useful information about the behavior of selected samples in various environments. In this work, spectroscopic technique was used for investigation of media effects on the linear optical properties of some drugs. The quantitative experimental results indicate that specific and nonspecific interactions with different contributions can modify the linear optical properties of folic acid, folinic acid and methotrexate drugs with different substituent in their chemical structures that these quantitative results can expand to the various groups of biological important compounds.
Keywords: Environment; Quantitative method; Linear optical properties; Drug
Introduction
During the last decades, the community needs for understanding of chemical structure and activity of various groups of drug molecules have been as an important challenge. It is interesting to know that recently scientists have developed the applicable analytical methods such as different types of spectroscopic technique [1-5] to study the unique characteristic of biologically important compounds. There is no doubt that provide of a proper method can give a precise information of complex molecular details. This is vital in disease diagnosis and design of pharmacologically and biological important compounds. In laboratory condition, the experimental researches and naturally occurring phenomena are usually used in solution state. Under this circumstance, solvent molecules have profound effects on the behavior of solute molecules in various biological, chemical and physical phenomena. In general, solvent sensitive processes are acquired from various molecular interactions, these includes general and specific solute-solvent interactions. Solvent induced effects are commonly discussed by their polarity and solvatochromic parameters [6-10]. The nature and strength of various molecular interactions cannot exactly have described by single solvent polarity parameters. Hence, multi-linear solvent polarity parameters known as Catalan equation (equation 1) are used [6].
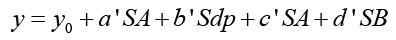
In above equation, y indicates under study solvent sensitive quantity. y0 is also describe the property of under study quantity in reference solvent or gas phase. a’, b’, c’, and d’ are obtained from multi-linear analysis that express the contribution of various solute-solvent interactions in various phenomena. The remained parameters in two equations are famous as solvent polarity scales. According to Catalan equation, SP, Sdp, SA and SB symbols describe solvent dipolarity, solvent polarizability, solvent acidity, and solvent basicity, respectively [11-12]. The aim of this experimental work is prediction of interactional behavior of biological important drug molecules quantitatively in solution state. Among of various groups of drugs, in this research, folic acid, folinic acid and methotrexate with vital role in various phenomena [13-18] are used due to their activity in various biological phenomena.
Experimental
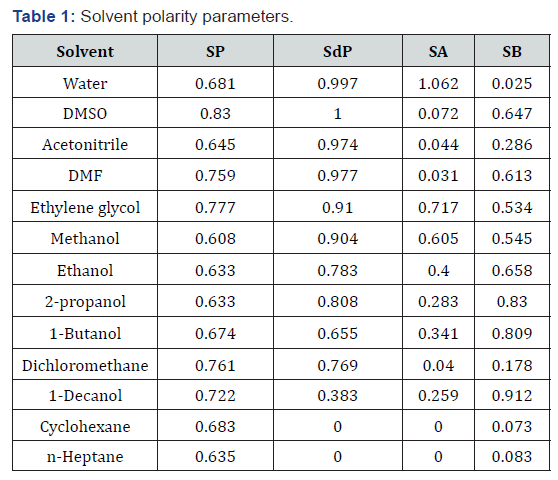
In this experimental work, in the first step, prepared solutions of folic acid, folinic acid and methotrexate with different polarity were poured in quartz cell while the experiment for each drug sample is repeated in different solvents. Then, the mentioned cell consist of drug molecules are placed in Double beam Shimadzu UV-240 Scan Spectrophotometer and JASCO FP-6200 Spectro fluorometer for recording of the absorption and emission spectra of selected samples, respectively. Moreover, all selected solvents are from Merck and solvent polarity parameters of them can be found in Table 1 [6].
Result and Discussion
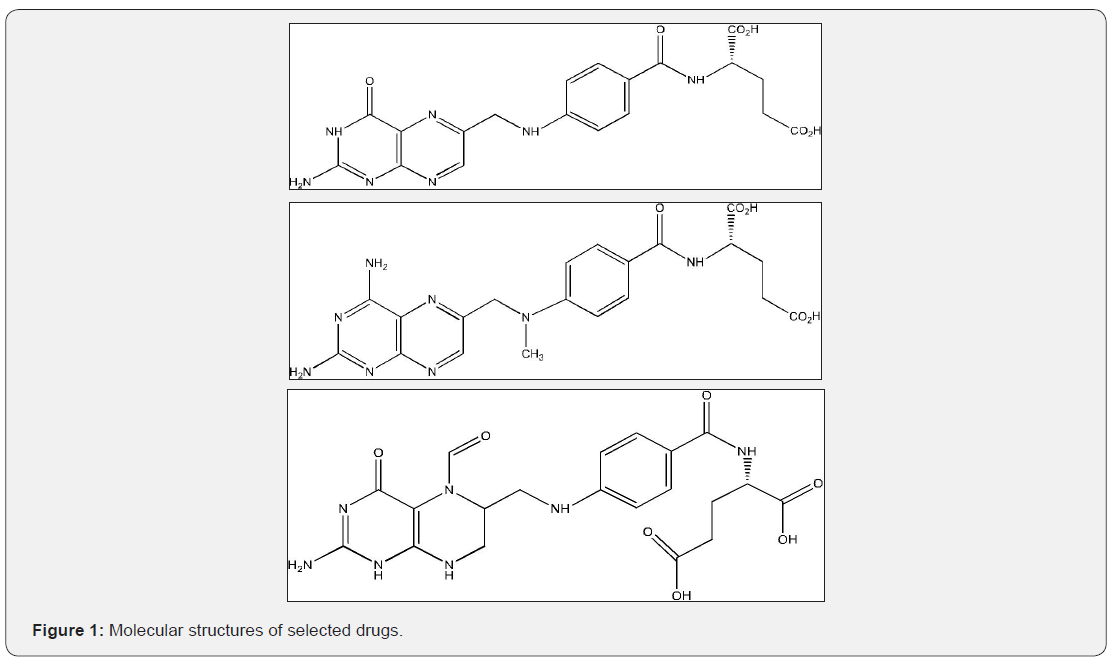
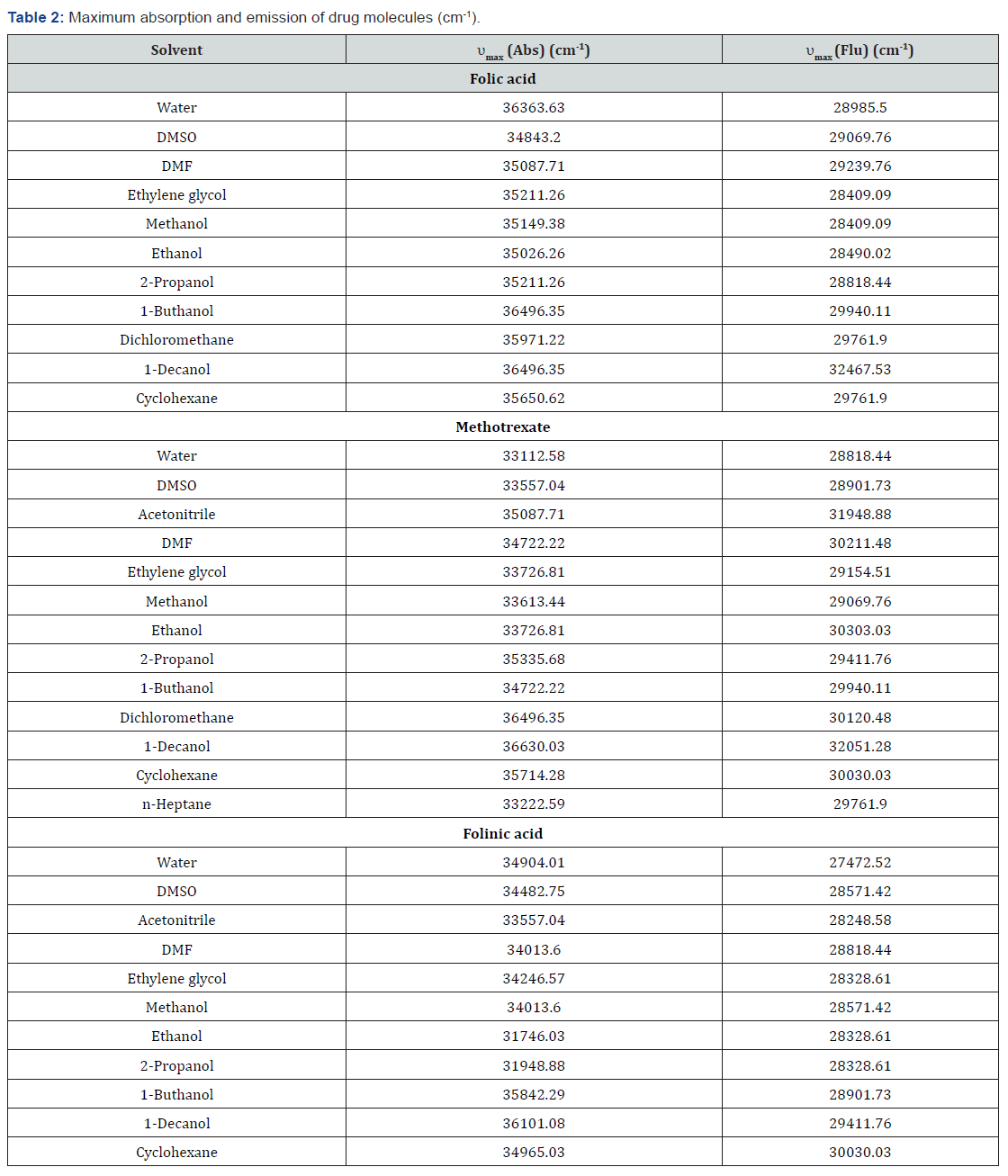
In this section, the interactional behavior of some drugs with different molecular structure (Figure 1) is investigated in various environments with different polarity. For this reason, the absorption and emission spectra of selected samples were recorded in UV-Visible region of electromagnetic spectrum at room temperature. The experimental data have provided in Table 2. According to these results, effective absorption and emission bands are in the regions of 274-300nm and 300-402nm of electromagnetic spectra. The results indicate that spectral shifts of drug samples by increasing media polarity are sensitive to presence of active groups in the molecular structure of folic acid, methotrexate and folinic acid drugs. In this case, maximum bathochromic spectral shifts in the ground state occur in folinic acid and maximum hypsochromic shifts occur in the excited state of Methotrexate by increasing media polarity. In tins case, various substituents lead to different molecular interactions between drug molecules and their surrounding media with different strength. Although this qualitative discussion can describe the positive or negative solvatochromic behavior of drug molecules, it cannot give sufficient information about nature and degree of various solute-solvent interactions.
In general, there are many unanswered questions about how drug molecules or other biological active molecules function in various solvent media especially in aqueous and alcoholic environments. By using a quantitative method, it is possible to investigate the interactional behavior of drug molecules in solution state and find dominant molecular interactions in solvent environments. In this case, correlation between the linear optical properties and solvent multi-parameter polarity scales will be useful. The results for three samples among of various groups of biological important compounds are formulated according to following equations for folic acid, methotrexate, and folinic acid, respectively.
Folic acid
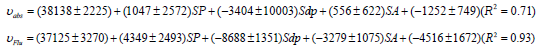
Methotrexate
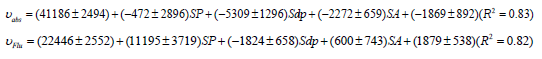
Folinic acid

Above equations give key information about interactional behavior of drug molecules and molecular stability in the ground and excited states. A precise studying indicates that in the ground and excited state of folic acid general media polarity effects have high contribution in the interactional behavior of drug molecules. In this case, in the ground and excited states, solvent polarizability plays significant role in the linear optical properties of folic acid. In addition, this dominant solvent polarity parameter tends to increase the stability of molecular excited state. The similar analysis for methotrexate indicates that although in the ground state, solvent polarizability has major role in optical properties of drug molecules, solvent acidity, basicity and dipolarity with lower contribution have minor role in the absorption properties of selected drug. In like behavior of drug molecule in the ground state, in the molecular excited, general solvent effects can be considered as important effects. In this case, an increment in values of dominant solvent polarity parameters can decrease the stability of ground and excited states of methotrexate.
The quantitative investigation of media polarity effects on the behavior of Folinic acid appears the interesting results. Under this circumstance, by transition to the excited state, the contribution of solvent polarizability and solvent acidity is increases. In addition, effective solvent polarity parameters enhance the stability of ground and excited states. Finally, by determination of contribution of general and specific solute-solvent interactions, the studying of function of biologically important molecules in various media with different polarity is facilitated. In this case, the effects of a small change in the polarity parameters of molecular surrounding media on the molecular function are easily detectable by provided quantitative equations. Moreover, the provided simple quantitative method is applicable for other compounds especially biologically sensitive molecules.
Conclusion
The present experimental work appears the interesting behaviors of three different groups of drugs in various environments with different polarity. Although a qualitative investigation can describe approximately some properties of under study molecules in different environment, it cannot describe exactly the contribution of various solute-solvent interactions. By determination of various molecular interactions contribution, it is possible to obtain useful information about how drug molecules or other biologically active molecules function in various media with different polarity. By this quantitative method, the effects of small changes in polarity of molecular surrounding media are detectable.
References
- Barth A, Haris PI (2009) Biological and biomedical infrared spectroscopy. IOS Press BV2, Netherlands, UK.
- Glencross H, Ahmed N, Wang Q (2011) Fundamentals of biomedical science. Oxford University Press, New York, USA.
- Ghomi M (2012) Application of Raman Spectroscopy to Biology (From Basic Studies to Disease Diagnosis). IOS Press BV5, Netherlands, UK.
- Merlin JC, Turrell S, Huvenne JP (1995) Spectroscopy of Biological Molecules, Springer Science+Business Media Dordrecht.
- Chen SH, Yip S(1974) Spectroscopy in Biology and Chemistry (Neutron, X-Ray Laser). Academic Press, New York and London, USA.
- Reichardat C (1988) Solvents and solvent effects in Organic chemistry, 2nd (edn),. Verlag Chemie Weinheim, New York, USA.
- Khadem Sadigh M, Zakerhamidi MS, Shamkhali AN, Babaei E (217) Photo-physical behaviors of various active forms of curcumin in polar and low polar environments. Journal of Photochemistry and Photobiology A: Chemistry 348: 188-198.
- Khadem M, Sadigh MS, Zakerhamidi B, Rezaei K, Milanchian (2017) Environment effects on the nonlinear absorption properties of Methylene blue under different power of excitation beam. Journal of Molecular Liquids 229: 548-554.
- Khadem Sadigh M, Zakerhamidi MS (2018) Media polarity and concentration roles on the third order nonlinear behaviors of thiazine dyes, Optics and Laser Technology 100: 216-224.
- Khadem Sadigh M, Hasani M, Rahimpour J (2018) Studying media polarity effects on the photo-physical behaviors of organometallic complexes with azo-containing Schiff-base ligands. Zeitschrift für Physikalische Chemie.
- Catalan J, Phys J, Chem B (2009) Toward a Generalized Treatment of the Solvent Effect Based on Four Empirical Scales: Dipolarity (SdP, a New Scale), Polarizability (SP), Acidity (SA), and Basicity (SB) of the Medium113(17): 5951-5960.
- Wypych G (2014) Handbook of Solvents, 2nd (edn),.
- Rubino FM, Separation methods for methotrexate, its structural analogues and metabolites. J Chromatogr B Biomed Sci Appl 764: 217- 254.
- Birdsall B, Casarotto MG, Andrew Cheung HT, Basran J, Roberts GCK, et al. (1997) The influence of aspartate 26 on the tautomeric forms of folate bound to Lactobacillus casei dihydrofolate reductase. FEBS Letters 402: 157-161.
- Chadha R, Arora P, Kaur R, Saini A, Lal Singla M, et al. (2009) Characterization of solvatomorphs of methotrexate using thermoanalytical and other techniques. Acta Pharma 59: 245-247.
- Mason JB, Levesque T (1996) Folate: effects on carcinogenesis and potential for cancer prevention, Oncology 10: 1727-1743.
- Choy JH, Jung JS, Oh JM, Parka M, Jeong J, et al. (2004) Layered double hydroxide as an efficient drug reservoir for folate derivatives, Biomaterials 25: 3059-3064.
- Hammud K, Mohammed JM, Radif MM, Raouf ALM, Mahmmood S, et al. (2013) Thermodynamic parameters for phenanthrene interaction with a biological p-acceptor (folic acid) by spectroscopicmeasurements. J Chem Pharm Res 5: 24-28.






























