Effect of Nickel Chloride on the Growth and Biochemical Characteristics of Phaseolus Mungol
K Selvaraj*
Department of Botany, Sri Kaliswari College (Autonomous), India
Submission: December 17, 2018; Published: April 24, 2018
*Corresponding author: K Selvaraj, Department of Botany, Sri Kaliswari College (Autonomous), Sivakasi Virudhunagar District, Tamilnadu, India, Tel: 97892406; Email: kselvarajphd@gmail.com
How to cite this article: K Selvaraj.Effect of Nickel Chloride on the Growth and Biochemical Characteristics of Phaseolus Mungol. JOJ scin. 2018; 1(1): 555556. DOI: 10.19080/JOJS.2018.01.555556
Abstract
The effect of heavy metal nickel chloride on germination, growth and biochemical parameters of Phaseolusmungo L. was studied. Application of various concentrations from 3mM to 15mM decreased the percentage of growth, pigment content and increased the content of amino acid and proline. It was found to be decreased when compared to the respective control grown with nutrient medium. The present study demonstrated that the heavy metal nickel had adversely affected the growth and biochemical parameters of the plant Phaseolusmungo L.
Keywords: Germination; Biochemical paramters; Heavy metal
Introduction
The accumulation of heavy metal in soil is becoming a serious problem as a result of industrial and agricultural practices and also a major cause for pollution today. Fertilizers from sewage sludge, mining waste and paper mills all contribute to the continuous deposition of heavy metals into soils. Another point of concern is the effect of leaching on these contaminated sites which in turn contaminate water tables [1]. Living organisms require a trace amount of some heavy metals which include copper (Cu), iron (Fe), nickel (Ni) and zinc (Zn) and are often referred to as essential elements [2]. However there are some non-essential heavy metals which are of great concern due to their presence in areas of heavy metal pollution such as chromium (Cr), mercury (Hg) and lead (Pb) [3]. The capacity of plants to concentrate metals has usually been considered a detrimental trait since some plants are directly or indirectly responsible for a proportion of the dietary uptake of toxic heavy metals by humans [4]. The dietary intake of heavy metals through consumption of contaminated crop plants can have long-term effects on human health [5].
Heavy metals are defined as metal with density higher than 5gcm3. Fifty three of the ninety naturally occurring elements are heavy metals and these are metallic elements which have a high atomic weight and density much greater than water. They are natural constituents of the earth' crust and are present in varying concentration in an all ecosystems. Based on their solubility under physiological conditions, seventeen heavy metals may be available for living cells and of importance for organisms and ecosystems. Among these metals, Fe, Mo and Mn are important as micronutrients. Zn, Ni, Cu, Co and Cr are toxic elements. As, Hg, Ag, Sb, Cd and Pb have no known functions as nutrients and seem to be more or less toxic to plants and microorganisms [6].
A plants response to heavy metal exposure varies depending on plant species, tissue and stage of development. Metal concentration and type of metal triggering is a series of defense mechanism which involve enzymatic and non- enzymatic components [1]. Plant tolerance to heavy metals depends largely on plant efficiency in the uptake, translocation, and further sequestration of heavy metal in specialized tissues or in trichomes and organelles such as vacuoles. The uptake of metals depends on their bioavailability, and plants have evolved mechanisms to make micronutrients bioavailable. Chelators such as siderophores, organic acids, and phenolics can help to release metal cations from soil particles, and also increasing their bioavailability. For example, organic acids (malate, citrate) excreted by plants act as metal chelators. By lowering the pH around the root, organic acids increase the bioavailability of metal cations. However, organic acids may also inhibit metal uptake by forming a complex with the metal outside the root. Citrate inhibition of aluminum (Al) uptake and resulting Al tolerance in several plant species is an example of this mechanism. Copper tolerance in Arabidopsis is also the result of a similar mechanism. The presence of rhizosphere microbes may also affect plant uptake of inorganics. For example, rhizosphere bacteria can enhance plant uptake of mercury and selenium. However, the exact mechanisms of these plant-microbe interactions are largely unknown. It is possible that the microbe mediated enhanced uptake will be either due to a stimulatory effect on root growth or to microbial production of metabolites that could affect plant gene expression of transporter proteins, or to a microbial effect on the bioavailability of the element.
Nickel (Ni) is an essential element that can be toxic and possibly carcinogenic in high concentrations. Ni is ubiquitously distributed in nature. It is found in different concentrations in all soil types of diverse climatic regions [7]. Naturally derived soils from serpentine rocks are rich in Ni, but due to various industrial and anthropogenic activities such as mining, refining of Ni ores, burning of fossil fuels and residual oil and sewage sludge, other areas have also become prone to Ni contamination [8]. The normal range of Ni in soil is 2 to 750ppm, with a critical soil concentration at 100ppm [9]. Exposure to Ni compounds causes irreversible damage to the central nervous system, cardiovascular system, lungs and gastrointestinal tract Nickel has been classified among the essential micronutrients and remains associated with some metallo-enzymes, but Ni is toxic at elevated concentrations in plants [7].
Nickel has a role in nitrogen metabolism that may stimulate plant growth and seed germination. In plants, Ni is responsible for chlorosis, yellowing and necrosis of leaves, deformation of plant parts, stunted growth and generation of free radicals [10]. One of the most persuasive ecological explanations for hyper accumulation of Ni and other toxic metals appears to be the defensive role against herbivores or pathogens. This function, which might be similar in other hyper accumulators, can be improved if the metal is localized in the outer layers of leaves and roots. Like in other Ni accumulators, such as Hybanthusfloribundus, Seneciocoronatus and Thlaspimontanum variety Siskiyouense, and A. bertolonii, Ni has been evidenced in leaf epidermal cells as a red stained nickel-dimethyl glyoxime complex [11,12]. Several Alyssum species are known to hyper accumulate nickel. These species can potentially be used to remediate Ni-contaminated soils.
At the same time there is convincing evidence of excess supply of nickel producing phytotoxic effects. Heavy metals accumulated in soil can affect flora, fauna and human livings in the vicinity of contaminated sites. The most of nickel is used to make stainless steel as a productive and ornamental coating for less corrosion. Nickel alloys are used in making coins and heat exchange items like valves. Nickel is combined with many other elements, including chlorine, sulfur and oxygen. Nickel compounds are used in plating, coloring ceramics making some batteries and as chemical reaction catalysts for dies, molds, cast propellers and valve seats. The problem of nickel toxicity acquires a series concern because of agriculture use of sewage sludge that is usually rich in nickel [13] and the industrial use of nickel production of Ni - Cd batteries which lead to discharge of nickel effluents.
Plant subjected to excess supply of nickel accelerates generation of toxic oxygen species leading to oxidative stress [14] and induces physiological water stress [15]. Excess nickel was reported to affect a number of biological and physiological processes resulting in an inhibition of plant growth [16,17].
Material and Methods
Seeds of Phaseolusmungo L. (Black gram) were procured from local seed centre, Thiruthangal. The healthy and viable seeds of Phaseolusmungo L. were surface sterilized with 0.1% of mercuric chloride for one minute and washed with running tap water followed by distilled water. The seeds were soaked in distilled water for 2 hours. Both control and experimental seeds were allowed to grow in plastic trough containing uniform amount of sandy soil. Seedlings were allowed to grow in half-strength of Hoagland nutrient solution for seven days. After seven days, the seedling were treated with different concentration of nickel chloride (3mM, 6mM, 9mM, 12mM and 15mM w/v) with half-strength of Hoagland nutrient solution, by keeping one trough without treatment as control. After seven days of metal treatment (on 15th day after germination) various morphometric and biochemical characters were analyzed.
For all morphometric characteristics, ten seedlings have been taken from both experimental and control sets and the results indicate the average of ten seedlings along with their respective standard error. The length of root of the randomly selected seedlings was measured with the help of meter scale. The length of the shoot of the randomly selected seedlings was measured for both control and experimental plants with the help of meter scale. The total leaf area of each and every plant was computed and expressed in cm2. The leaf area of the harvested leaves was measured by conventional graphical method. The fresh weight of the seedlings was obtained using an electronic balance soon after harvest. Care was taken to avoid wilting of plant parts. The fresh undamaged seedlings were kept in an oven at 70 °C for 24 hours. After complete drying, the seedlings were weighed using an electronic balance.
For all biochemical analysis the average of 5 samples were taken from both control and treated plants separately and the results indicate the average of five seedlings along with their respective standard error. To extract the total chlorophyll from leaves, fresh leaves were deveined and cut into small bits. From the pooled leaf bits, a sample of 100mg was weighed. The leaf bits were homogenized in 100% acetone using a mortar and pestle. The homogenate was centrifuged at 4000rpm for 5 minutes at room temperature. Extraction with 100% acetone was repeated until the pellet becomes pale yellow or white in colour. The supernatant was used for the estimation of photosynthetic pigments. The absorbance was measured at 662nm, 645nm and 470nm for chlorophyll a, chlorophyll b and Carotenoids, respectively using ELICO SL 171 Spectrophotometer. The amount of chlorophyll a,b and total chlorophyll was calculated by using the formula of Wellburn and Lichtenthaler.
The protein content of the leaf tissue was measure by Lowry's method (1951). Fresh leaf sample (100mg) was ground in 10ml of distilled water with the help of mortar and pestle. The homogenate was centrifuged at 5000rpm for 5minutes and the supernatant was added with 1 ml of ice cold TCA and again it was centrifuged. The pellet was dissolved with 1ml of 0.1 N NaoH and it was used as test solution. From the test solution, 0.1ml was taken in the test tubes and it was added with 0.5ml of distilled water, 5.5ml of alkaline copper mixture and 0.5ml of Folin phenol reagent. It was mixed thoroughly and kept in condition for 10 minutes to develop blue color. The absorbance was noted at 650nm using ELICO SL 171 Spectrophotometer. The protein content was calculated from the standard graph of protein constructed with bovine serum albumin as marker protein.
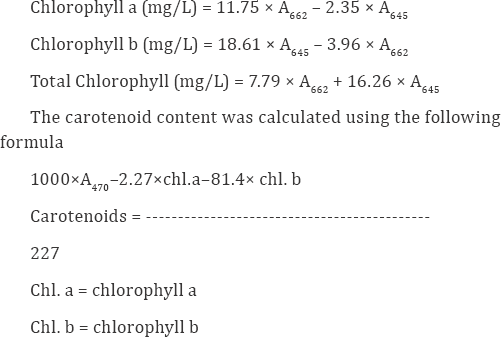
Total soluble sugars in leaves were estimated by Anthrone method. 100 mg of fresh leaves of both control and treated plants were ground in 10ml of distilled water using mortar and pestle. The homogenate of leaves was centrifuged at 3000rpm for 5minutes. The supernatant was taken, it was added with 2ml of 10% TCA and kept in the ice cold condition for 10 minutes, and again it was centrifuged at 5000rpm for 5minutes. The supernatant was used as test solution. 0.1ml of test solution was taken in test tubes and it was added with 0.9ml of distilled water and 4ml of Anthrone reagent (0.2%). The test tubes were boiled in water bath for 10minutes after cooling; the absorbance was measured at 620nm. The amount of sugar present in the extract was calculated from a standard curve using glucose as the standard (Figure 1).
Free amino acids were estimated by Ninhydrin assay method. The leaf material (100mg fresh weight) was ground in 10ml of ethanol. The homogenate was centrifuged at 5000rpm for 3minutes. The pellet was discarded and the supernatant was used as test solution. 1ml of test solution, 3ml of distilled water and 1ml of Ninhydrin reagent were added and mixed thoroughly. After mixing, the test tube was kept in boiling water bath for 10minutes. Then the tube was cooled down to room temperature and 1ml of 50 % ethanol was added. The absorbance was measured at 550nm using proper blank. Blank solution consisted of 4ml of distilled water, 1ml of Ninhydrin reagent and 1ml of ethanol. The amino acid content was estimated from standard curve prepared with glycine as amino acid source.
Proline content was estimated according to. The 100mg leave sample were ground in 3% (w/v) Sulphosalicylic acid. The extract was filtered through Whatmann No.1 filter paper. 2ml of the extract, 2 ml of acid Ninhydrin (1.25g of Ninhydrin in a mixture of 30ml of glacial acidic acid and 20ml of 6M Phosphoric acid) and 2ml of glacial acidic acid were added. The contents were shaken well and the tubes were kept in the water bath at 100 °C for 1hour. After 1hour the tubes were allowed to cool down to room temperature and then kept in ice for 5minutes, to terminate the reaction. The 4ml of toluene was added and the tubes were agitated vigorously and then allowed to stand. The proline containing chromophore was aspirated and the absorbance was read at 520nm. The proline content was calculated from a standard curve prepared authentic sample of proline.
Statistical Analysis
Morphometric parameters were determined with ten independent replicates. Biochemical characters were carried out at least five times. The data were reported as mean±SE and in parentheses represent the percent activity.
Results
The results obtained on the effect of different concentration of nickel chloride and nickel + seaweed liquid extract treated plants were given as follows. Impact of various concentration of nickel chloride on the morphometric characteristics of Phaseolusmungo L. is shown in graph 1. With the increase in concentration of nickel chloride root length were slowly decreased ranging from 12% to 45% compared to control. The minimum root growth was found in 15mM nickel concentration. Shoot length also followed a similar declining trend where the reduction was 44% in higher concentration of nickel chloride. The leaf area was gradually reduced with increasing concentration of nickel chloride. The reduction was about 37% in 15mM concentration of nickel chloride. With increase in concentration of nickel chloride the fresh weight was found to be decreased. The minimum fresh weight was observed in 15mM Nickel chloride treated seedlings. Total plant biomass accumulation (dry weight) is a good indicator of any stress study. The dry weight was analyzed in nickel treated Phaseolusmungo L. seedlings. It showed significant reduction than the control plants.
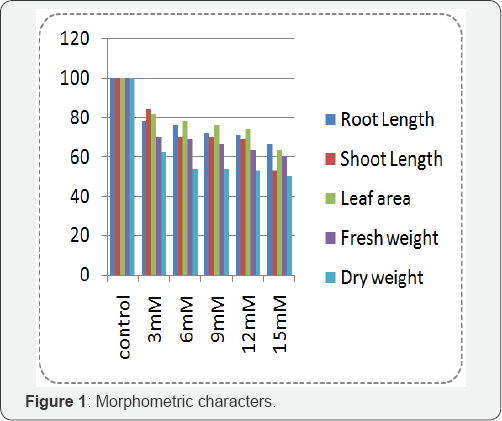
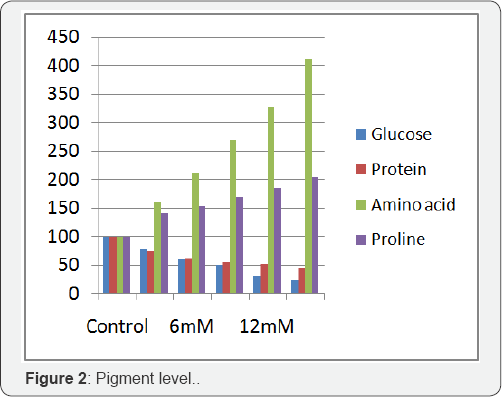
Results obtained on the impact of various concentration of Nickel chloride on the photosynthetic pigments of Phaseolusmungo L. is shown in Figure 2. Pigment content also showed the declining trend with increasing concentration of nickel chloride. The chlorophyll content of nickel chloride treated Phaseolusmungo L. showed considerable reduction over control plants. The maximum reduction of chlorophyll pigment brought about by 15mM concentration to the level of 67% and the carotenoid was found to be 78% in 15mM concentration of Nickel chloride.
Result obtained on the impact of various concentration of nickel chloride on the biochemical characteristics of Phaseolusmungo L. is shown in Figure 3. The sugar content was decreased with the increase in the concentration of nickel chloride. At 15mM concentration of nickel chloride, the reduction was about 76% less than the control. The protein content was found to be decreased with the increase in the nickel concentration on experimental plants. At 15mM concentration of nickel chloride the reduction of protein content was 55% less than the control plants. The nickel chloride had a considerable increase in the free amino acid content of the nickel treated plants than the control plants. The free amino acid content increased from 60% to 311% when compared to the control. The proline content increased with the increasing concentration of nickel chloride.
Discussion
Results obtained on the morphometric and biochemical character of black gram Phaseolusmungo L .treated with nickel chloride and nickel + seaweed extract are discussed below. The result obtained on the present study indicate that, increase in concentration (3mM to 15mM) of nickel chloride, showed a decrease in root length, shoot length, fresh weight and dry weight of Phaseolusmungo L .Decrease in shoot length, root length, fresh weight and dry weight may be due to nickel toxicity which caused reduction in water uptake [18,19].
The observed pronounced inhibition of shoot and root growth and leaf area are main cause for the decrease in fresh weight and dry weight of seedlings. For plants, uptake of metals occurs primarily through the roots, so this is the primary site for regulating their accumulation [20]. The reduction in leaf area in response to nickel treatment was also related to accumulation of nickel in leaves, where the size of the leaf was also decreased. This result coincides with the findings of Panday & Pathak [21], where the leaves of green gram plants supplied with excess nickel were smaller in size and developed chlorosis in the leaflets. After seven days of nickel supply, these leaves developed black necrotic spots on either side of the midrib.
The photosynthetic process is related with the inhibition of biomass accumulation, which in turn relies upon the pigment level. The chlorophyll content, which is an indicator of the photosynthetic activity of plants, showed a remarkable reduction in nickel treated plants. Plants subjected to nickel toxicity showed decrease in the concentration of chlorophyll and carotenoids. The decrease in these plant pigments may be due to cellular disorganization under nickel toxicity which causes agglutination of chloroplast.
Under the heavy metal sodium chloride treatment also there was a considerable reduction in growth and photosynthetic pigments; this may be due to the disturbance in photo system I and chlorophyllase enzyme. This disturbance paralleled with the reduction in sugar content may be attributed to reduction in chlorophyll contents of the leaf and also a decline in protein. This change might have already affected the photosynthetic activity in the plant and hence the reduction in carbohydrate contents [22,23].
Satyakala & Jamil [24] observed a reduction in the protein contents in the roots, leaves and petioles of water hyacinth and lettuce plants after chromium treatment and suggested that metal ions seems to interfere with protein synthesis which is one of the major components for biochemical activities. In the present study a reduction in protein content observed in nickel chloride treated plants, may attributed to the decrease in the synthesis of protein macromolecules under nickel toxicity and the denaturation of protein by protease activity resulting in increasing level of protein degradation.
As a result of protein degradation during stress condition, the availability of free amino acid is significantly high. The free amino acid content is increased with the increase in nickel supply. It may be due to destruction of protein or to the biosynthesis of amino acid from the nitrate source which were not utilized in the protein synthesis [25]. It is an adaptative mechanism by the plant cell to overcome post stress metabolism [26]. Accumulation of proline has frequently used as a biochemical marker for water stress in plants [27,28]. The reduction of stress in plants has thought to promote the accumulation of proline and to act as a cytoplasmic osmotic solute [29,30]. L-Proline accumulation may cause by stimulated synthesis from glutamate, slower incorporation of proline into protein and failure in protein synthesis. Proline accumulation is considered a protective device for the plants to preserve water, which is necessary to tide over any internal water deficit situation. The accumulation of proline is also considered as an adaptive response to stress [31].
The possibility of proline accumulation is because of the impaired protein synthesis. In a stress condition the inhibition of growth of cells, leaves and the whole plant is accompanied by an accumulation of nitrate in plant tissue particularly in leaves [32]. The leaf nitrate content found to be more in treated plants. The accumulation of leaf nitrate in the present study was found to be paralleled with the reduction in nitrate reductase (NR) activity.
Thus, nickel caused a reduction in photosynthetic pigment content, which was paralleled with reduction in photosynthetic product called sugar. Reduction in total soluble protein in the leaf could also be ascribed to the reduction in photosynthesis. Accumulation of free amino acid indicates the degradation of protein in all the nickel treated plants, proline an osmotic regulator accumulate more in all the treated plants [33,34]
References
- Gratao PL, Prasad MNV, Cardoso PF, Lea PJ, Azevedo RA, et al. (2005) Phytoremediaion: green technology for the clean up of toxic metals in the environment. Braz J Plant Physiol 17: 53-64.
- Odjegba VJ, Fasidi IO (2004) Accumulation of Trace Elements by Pistiastratiotes: Implications for phytoremediation. Ecotoxicology 13: 637-646.
- Kamal M, Ghaly AE, Mahmoud N, Cote R (2004) Phytoaccumulation of heavy metals by aquatic plants. Environment International 29: 10291039.
- Chaney RL, Malik M, LI YM, Brown SL, Brewer EP, et al. (1997) Phytoremediation of soil metals. Curr Opin Biotechnol 8: 279-284.
- Ow DW (1996) Heavy metal tolerance genes: prospective tools for bioremediation. Resources Conservation Recycling 18: 135-149.
- Godbold DL, Huttermann A (1985) Effect of cadmium mercury on root elongation of Piceaabies (Karst.) Seedlings and the significance of these metals to forest dieback. Env Pollution 38: 375-381.
- Srivastava S, Mishra S, Dwivedi S, Baghel VS, Verma S, et al. (2005) Nickel Phytoremediation Potential of Broad Bean, ViciafabaL., and Its Biochemical Responses. Bull Environ Contam Toxicol 74: 715-724.
- Nriagu JO (1988) Production and uses of chromium. Chromium in natural and human environment. John Wiley and Sons, New York, USA.
- Gardea-Torresdey JL, Peralta-Videa JR, De La Rosa G, Parsons JG (2005) Phytoremediation of heavy metals and study of the metal coordination by x-ray absorption spectroscopy. Coordination Chemistry Reviews 249: 1797-1810.
- Halliwell B, Gutteridge JMC (1999) Free Radicals in biology and medicine, Oxford, UK.
- Martens SN, Boyd RS (2002) The Defensive Role of Ni Hyper accumulation By Plants: A Field Experiment. American Journal of Botany 89: 998-1003.
- Leon V, Rabier J, Notonier R, Barthelemy R, Moreau X (2005) Effects of Three Nickel Salts on Germinating Seeds of Grevilleaexul var. rubiginosa, an Endemic Serpentine Proteaceae. Annals of Botany 95: 609-618.
- Juste C, Mench M (1992) Long term application of sewage sludge and its effect on metal uptake by crops. In: Adriano DC (Edt.), Biogeochemistry of trace metals, Ann Arbor, Tokyo, Leuwis publishes, London, pp: 159193.
- Schiclker, Caspi (1999) Responses of antioxidative enzymes to nickel and cadmium stress in hyper accumulator plants of genus Alyssum. Physiol Plant 105: 39-44.
- Panday N, Sharma CP (2002) Effect of heavy metals Co2+, Ni2+ and Cd2+ on growth and metabolism of Cabbage. Plant Sci 63: 753 -758.
- Jones MD, Hutchinson TC (1998) Nickel toxicity in mycorrhizal birch seedling infected with Lactariusrufus or Scleroderma flavidum. I. Effect on growth, photosynthesis, respiration and transpiration and transpiration. New phytol 108: 451-459.
- Gajeswska E, Slaba M, Andrezejewska R, Sklodowska M (2006) Nickel induced inhibition of wheat root growth is related to H2O2 production, but not to lipid peroxidation. Plant Growth Regul 49: 95-103.
- Foy CD, Chaney RL, White MC (1998) The physiology of metal toxicity in plant. Annu plant physiol 29: 551-556.
- Nag P, Paul AK, Mukherji S (1981) Heavy metal effects in plant tissues involving chlorophyll, chlorophyllase, hill reaction activity and gel electrophoretic patters, of soluble proteins. Indian J Exp Biol 19: 707709.
- Arduini I, Godbold DL, Onnis A (1996) Influence of copper on root growth and morphology of Pinuspinea L. and Pinuspinaster Ait Seedlings. Tree physiology 15: 411-415.
- Panday N, Pathak GC (2006) Nickel alters anti oxidative defense and water status in green gram. Indian J Plant Physio 11 : 113-118.
- Swaminathan K, ArjunanJ, Gurusamy R (1998) Effect of glucose factory effluents on the seed germination and seedling development of groundnut (Arachishypogea L.) Envirion Biol 2: 187-189.
- Dowton WJS (1977) Photosynthesis in salt stressed in grape wines. Aust J Plant Physiol 4: 183-192.
- Satyakala G, Jamil K (1992) Chromium induced biochemical changes in Eichhorniacrassipes, Mart and Pistiastratioses L. Bull Env ContamToxicol 48: 921-928.
- Sharma SK, Srivastava A, Singh VP (1997) Effect of rubber factory effluent on growth in Vignamungo J Environ Poll 4: 175 -177.
- Singh DS, Vijayakumar KP (1974) Carryout the effect of salinity on yield and quality of wheat seed. Seed Res 21: 13-18.
- Alia P, Saradhi PP (1991) Proline accumulation under heavy metal stress J Plant Physiol 138: 534-538.
- Schat H, Sharma SS, Voojis R (1997) Heavy metal induces accumulation of proline in metal tolerant and non-tolerant ecotypes of Silene vulgaris. Physiol Plant 101: 477-482.
- Levy D (1983) Water deficit enchances praline and alpha - amino nitrogen and its association with susceptibility to drought. Plant Physiol 551: 169-173.
- Newton RJ, Sen S, Puryer JD (1986) Free proline changes in Pinustaedas L. Callus in response to drought stress (growth, water potential desiccation tolerance).Tree Physiol 41: 325-332.
- Lee KG, Gunningam BA, Paulsen GM, Liang GH, Moore RB (1976) Effect of cadmium on respiration rate and activities of several enzymes in soybean seedlings .Physio Plant 36: 4-6.
- Sinha, Nicholas DJD, Paleg LG, Aspinall D (1981) The physiology and biochemistry of drought resistance in plants. Academic Press, London, pp: 145-169.
- Baccouch S, Chaoui A, El Ferjani E (1998) Nickel induced oxidative damage and antioxidant responses in Zea maize shoots. Plant Pysiol Biochem 36: 689-694.
- Gaurav K, Dinabandhu S (2011) Effect of seaweed liquid extract on growth and yield of Triticumae stivum var. Pusa Gold, Journal of Applied Phycology Volume 23: 251-255.






























