Can Impedance Eye Recording (IER) be the New Health’s Eye Testing?
Pierre-Alain Grounauer1*, Cloé Houriet2 and Marie-Valerie Moreno3
1University Eye Clinic, Lausanne, Switzerland
2Fabrinal SA, rue de la tuilerie, 2300 La Chaux-de-fonds, Switzerland
3HzCare, 6 allée Jean Chorel, Sathonay-Village, France
Submission:June 28, 2024; Published:July 18, 2024
*Corresponding author: Pierre-Alain Grounauer, University Eye Clinic, Lausanne, Switzerland Email: pagrounauer@bluewin.ch
How to cite this article: Grounauer Pierre-Alain, Houriet Cloé, Moreno Marie-Valerie. Can Impedance Eye Recording (IER) be the New Health’s Eye Testing?. JOJ Ophthalmol. 2024; 11(2): 555809 DOI: 10.19080/JOJO.2024.11.555809
Abstract
Impedance eye recording (IER) is a new electrophysiological method for testing many parameters in the anterior and posterior eye chamber. Continuing our previous work, we seek in this study on humans to validate the first hypotheses concerning the feasibility of the system (viable repeatability, an estimation of the intraocular pressure possible by bioimpedance, an estimation of the thickness of the cornea and the permeability of the trabecular meshwork for early detection of glaucoma, the establishment of reference tables). 30 participants, by whom both eyes will be measured: age between 18 and 30 years old, with healthy eyes and no history of eyes diseases. We obtained a viable repeatability globally less than 1 ohm. We noticed none significatively difference between our estimation of intraocular pressure and estimation of corneal thickness comparatively to the reference (p-value Wilcoxon > 0.1). This study allowing us to open a new chapter in ophthalmic eye evaluation functions. Further studies need to be projected.
Keywords:Impedance Eye Recording (IER); Intraocular Pressure (IOP); Corneal Thickness; Trabeculum Permeability; Posterior Chamber; Ophthalmic Vascular Zone; Oxymetry
Abbreviations: IER: Impedance Eye Recording; IOP: Intraocular Pressure; CV: Coefficient of Variation
Introduction
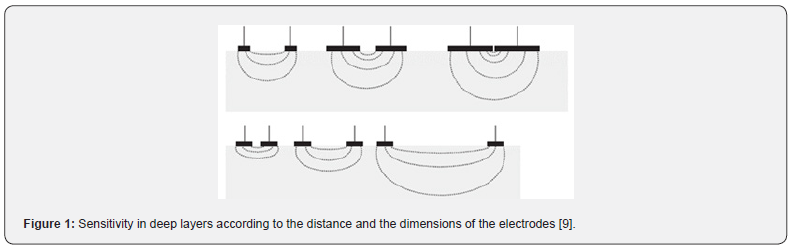
Biological measurements using electrical impedance technology on living organisms are not new. They are frequently used on isolated living vegetable cells or animal cells organized into organs of the human body, notably in pneumology and cardiology [1-2]. On the human eye [3-8], we have shown that this technique can also be used without causing tissue damage, providing original, clinically useful results. Thanks to the development of microelectronic bioinformatics, our team has designed a specific new module. This generator of amplitude- and frequency-modulated electrical micro-signals makes it possible to interrogate cellular responses to these currents and visualize them in the form of Cole-Cole curves in 2D. To measure these signals, electrodes are applied to the patients. Grimnes et al. [9] explain the relationship between the sensitivity of the measure according to the depth of tissue being studied and the increase distance between the electrodes and/or the increase in surface area of the electrodes (Figure 1).
It is in this context that we placed the electrodes in the lens to access the anterior chamber of the eye.
According to Kanai and Meijer [10,11], the cell membrane behaves like a capacitor. Indeed, a membrane is made up of phospholipids and is therefore insulating. When current encounters a cell membrane, electrical charges build up on both sides of the membrane without being able to pass through it. An electric double layer is then created, which forms a capacitor. A reactance X (ohm) can be associated to this phenomenon (Equation (1)). These data vary according to the frequency.
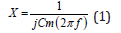
where X is the reactance (Ω), f the frequency of the injected current in Hz and Cm the capacity of the membrane in Farad.
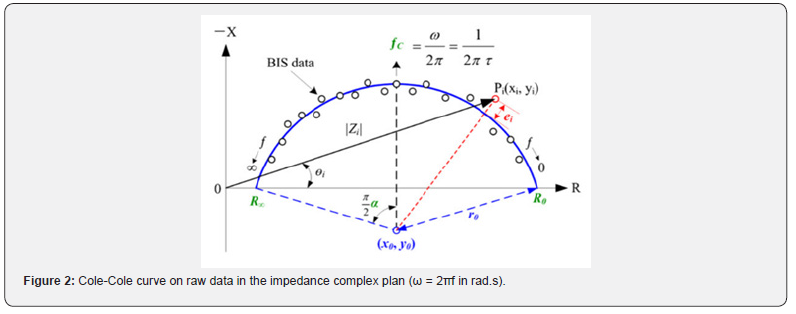
In that model, the extracellular resistance is Re (obtained to a theoretical f = 0 Hz) and the total resistance of the tissue is R∞ or Rinf (obtained to a theoretical infinite frequency). The impedance (the sum of the resistive and capacitive properties) of the tissue measured is then a complex number which can be graphed in a complex plan (the imaginary part of the impedance Zimg versus the real part Zr). As described by Bonnet et al. [12], we can fit a Cole–Cole curve (Figure 2) to the raw data to obtain new parameters. Unlike the authors, we do not use the Kasa method, but the nonlinear least squares method. Those parameters characterize biological tissues.
Continuing our previous work [8,13,14], we seek in this study on humans to validate the first hypotheses concerning the feasibility of the system:
i. Viable repeatability
ii. An estimation of the intraocular pressure possible by bioimpedance
iii. An estimation of the thickness of the cornea and the permeability of the trabecular meshwork for early detection of glaucoma
iv. The establishment of reference tables.
Materials and Methods
Population and Protocol
30 participants, by whom both eyes will be measured: age between 18 and 30 years old, with healthy eyes and no history of eyes diseases, flat corneal radius (7.5-7.8 mm), corneal eccentricity (0.5-0.7), astigmatism (<0.3 mm). Due to wire crossing, only 12 on 30 has been statistical enclosed for the results on anterior chamber. The exclusion criteria were: systemic disease that may affect ocular health, such as diabetes, ocular disease, injury and history of ocular surgery, and allergy to topical anaesthesia with oxybuprocaine (0.4%).
Participant recruitment occurs via advertisement at the Institute of optometry (FHNW, Olten, Switzerland) within the patient pool of the optometry clinic, either via email, or by posting an advertisement at the black board in the entrance area of the clinic. Screening for fulfilment of inclusion criteria occurs via topographic measurements of the cornea with the OCULUS Keratograph. The examiner will explain to all participating participants the relevant study contents, study aims, procedures, expenditure of time, benefits, and financial participant compensation. He will assure that study participation is always voluntary and those participants can terminate their participation at any time without providing any reasons.
This information will be given to all participants in written form one week before study commencement. This ensures that all participants will be given sufficient time to read all the information given and to decide on study participation. The formal consent of a participant, using the approved informed consent form, must be obtained before the participant is submitted to any of the study procedures. Participants will read and consider the statement before signing and dating the informed consent form and will be given a copy of the signed document. The consent form will also be signed and dated by the examiner at the same time as the participant signs it, and the original will be retained as part of the study records. This protocol was approved by Swiss Ethics.
Material
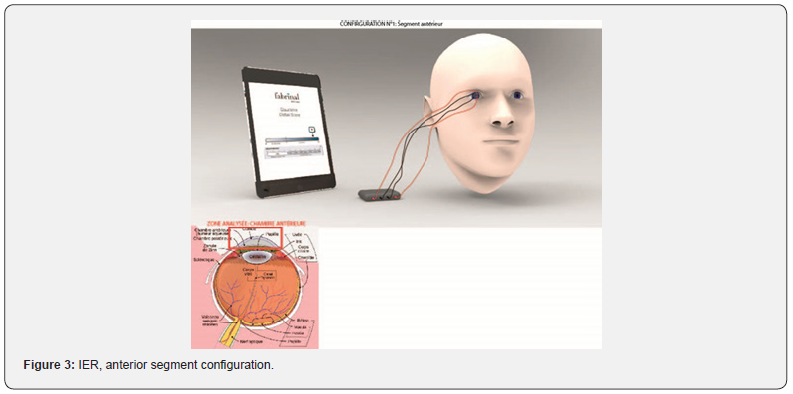
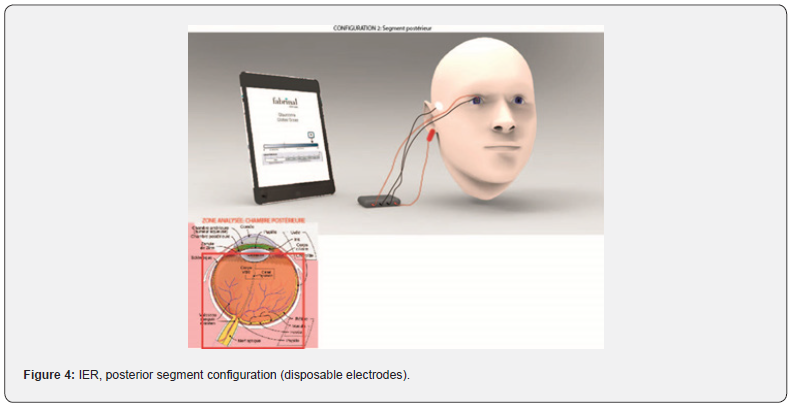
Impedance Eye Recording (IER) is composed by the electronics board from RunSys and the ICE-bi (impedance corneal electrode) single use and sterile electrode, from Fabrinal. The device allows the analysis of the anterior chamber (Figure 3). Moreover, it allows the analysis of the full eye, and therefore by deduction of the posterior chamber (Figure 4). In that case, we add disposable sterile electrodes Ag/AgCl 3M on the temple and/or the earlobe.
Lens (Fabrinal SA)
The sensor for the ICE-bi lens (Figure 5) is based on the design of the ERG-jet lens. The ERG-Jet is a contact lens incorporating an electrode that captures the electroretinogram, ERG, generated by light stimulation of the human or animal retina. It represents a plastic lens with a concave shape. A thin gold ring inside the lens allows the detection of the electrical signal. A conductive wire transmits the electrical signal to a preamplifier and a data processing device. The ERG-Jet is used to support diagnostic systems for eye abnormalities by transmitting the retinal electrical signal. (The ERG-Jet is also popular in the veterinary field due to its simplicity and small footprint compared to some competing products. Animal use is outside the scope of the medical device according to MDD 93/42 and MDR).
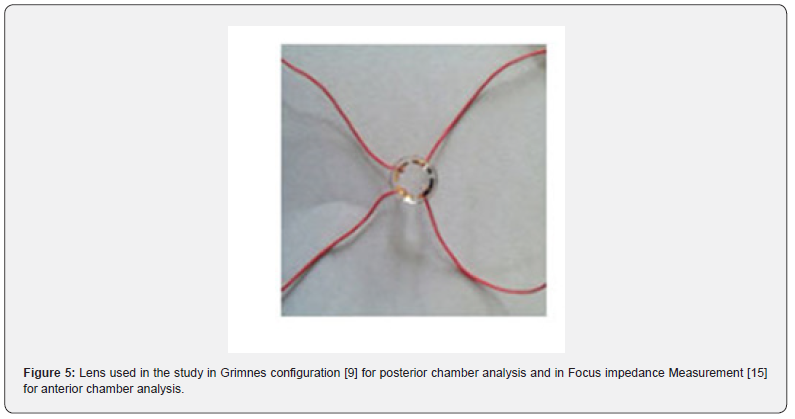
F-06 LENS, SPECIFICATION: Diameter: 12 mm (1/2 ‘’), Cable length: 1000 mm (39 ‘’), Bending radius: 7.90 mm, Female connection: DIN 1.50 mm (Design: P. A. Grounauer M.D).
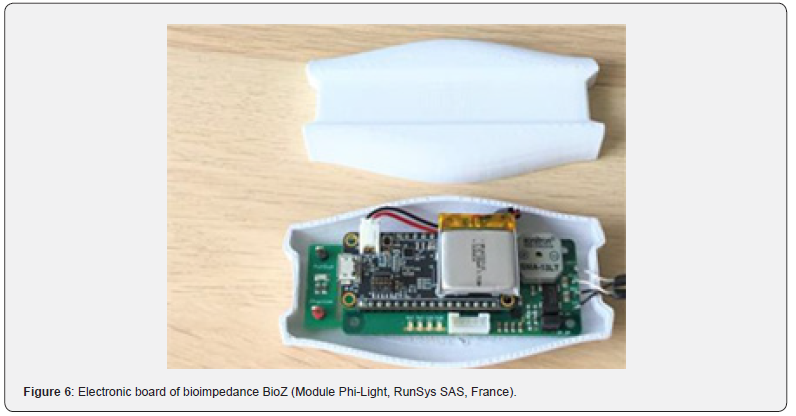
Bioimpedance Module (RunSys SAS)
Impedance measurements (Resistance R, Reactance X and Impedance Z) are performed with a bioimpedance electronic board (ZRange (0-2500 ohms); Zreal Coefficient of Variability (CV) % 0.025% and Zimg CV% 0.408%; accuracy (all frequencies) Zr mean error 2.60% ± 2.01% and Zimg mean error 0.43% ± 0.84%) (Figure 6).
Bioimpedance applied to the eye consists of injecting a very low intensity (in the order of 32 micro- amperes) and high frequency (from 4 to 128 kHz) current through electrodes. The injection of micro-currents into the eye is safe based on comparison with iontophoresis which uses currents 1000 times greater and for longer time (15 seconds compared to more minutes) than those of IER.
The electronic measurement chain was validated by the French National Agency on Medical devices (ANSM) for 2 others clinical trials with the following IDs: ID RCB 2022-A01524-39 and ID RCB 2022-A00677-36.
Methods
To ensure measurement repeatability, we calculated the coefficient of variation (CV) for each raw signal using Equation (2):
VariationCoefficinet (CV)%=(Standard Deviation Median) x100 (2)
The significance of the estimation of intraocular pressure (mmHg) and the estimation of corneal thickness (mm) were analyzed (after verifying data normality by a Shapiro-Wilk test) by applying non parametric paired Wilcoxon signed ranks tests to derive p-values for significance levels (first grade of significance *, p < 0.05; second grade **, p < 0.01 and third grade ***, p < 0.001).
Two dedicated algorithms were developed to estimate intraocular pressure (mmHg) and the estimation of corneal thickness (mm), using a simplified logistic binomial law (two parameters μ, s) Equation (3), with its density and distribution functions calculated as shown in Equations (4) and (5), respectively.
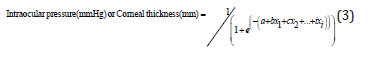
where a, b, …, i are constants and x1, x2, …, xi are experimental variables of the equation.
In this study, having fewer experimental variables, we used the simplified logistic binomial law (two parameters μ, s), with its density function calculated as Equation (4):
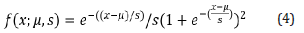
Then, its distribution function is calculated as Equation (5):
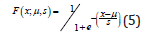
We notice in our study the Esperance E(X) = μ=0 and the variance Var (X) = s²π²/3, inducing s = 1.
Then, the obtained distribution function of the water status score is calculated as Equation (6):
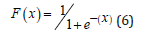
with x corresponding to the absorbance characteristics measured.
The algorithm was initially created with 6 observations and later validated on an independent subgroup of 6 observations.
Results
Repeatability
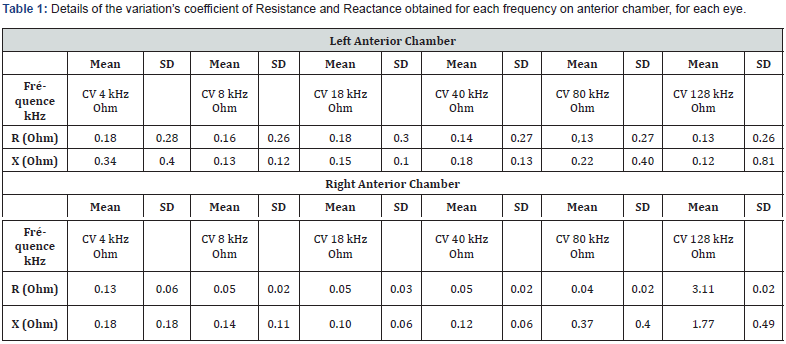
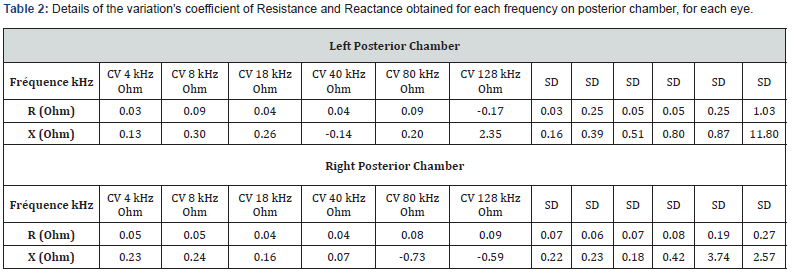
Table 1 details the variation’s coefficient of Resistance and Reactance obtained for each frequency on anterior chamber, for each eye. The repeatability of measurements on the anterior chamber is acceptable with a variability of less than 1 ohm on average, whatever the frequency. On the right eye, we note a variability greater than 128 kHz (Hook effect). Table 2 details the variation’s coefficient of Resistance and Reactance obtained for each frequency on posterior chamber, for each eye. The results are similar in Post configuration. There is a significant standard deviation in the variability of the right eye at 80 kHz due to a particular subject.
Estimation of Intraocular Pressure (mmHg)
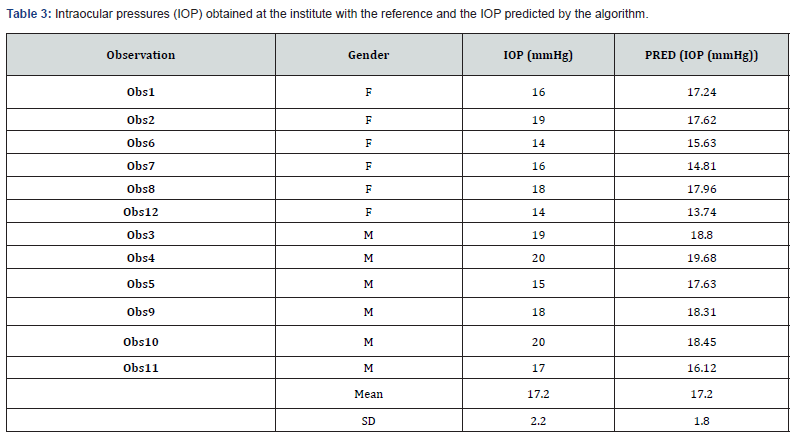
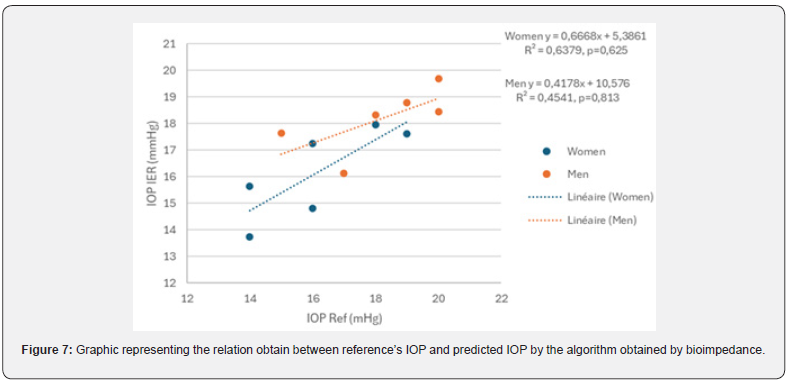
Table 3 details these intraocular pressures (IOP) obtained at the institute with the reference and the IOP predicted by the algorithm. Estimated IOP is not significantly different from the reference (p (Wilcoxon)> 0.1). Figure 7 highlights a difference between man (R² = 0.454) and women (R² = 0.638).
Estimation of Corneal Thickness (mm)
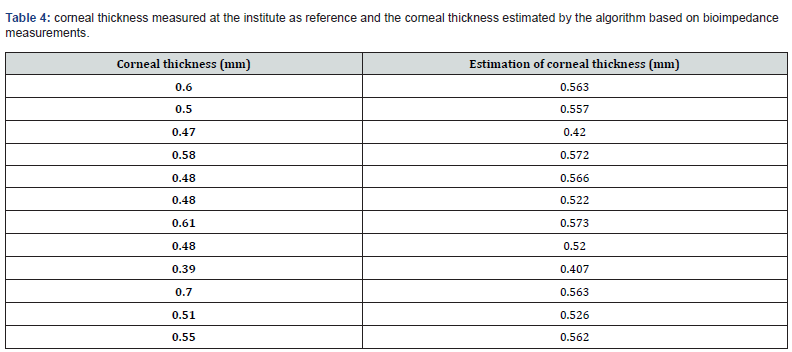
Table 4 details the corneal thickness measured at the institute as reference and the corneal thickness estimated by the algorithm based on bioimpedance measurements. Corneal thickness is not significantly different from the reference (p(Wilcoxon)=0.677>0.1).
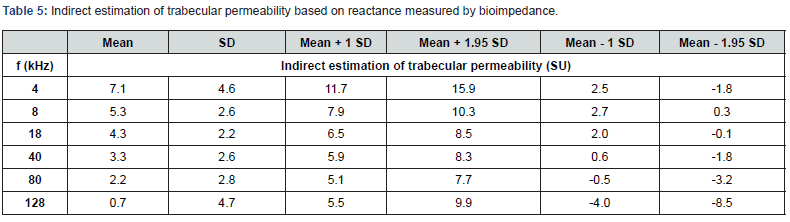
Indirect Estimation of Trabecular Permeability (SU)
Table 5 present the indirect estimation of trabecular permeability based on reactance measured by bioimpedance. We observe that trabecular cells have on average a phase angle of the same order of magnitude as other cells in the organism. In view of the importance of the extracellular mass in the trabecular meshwork. we can think that the phase angle at 4 kHz could be an interesting biomarker. These first reference tables on healthy subjects could make it possible to detect any dysfunction of the trabecular meshwork.
Reference Tables of Electrical Parameters on Human Eye (Resistance and Reactance)
These first reference (Tables 6 and 7) on healthy subjects could make it possible to detect fluid dysfunction, particularly in the anterior chamber or certain cells present (such as the cornea). These first reference tables on healthy subjects could make it possible to detect fluid dysfunction, particularly in the posterior chamber. of the ophthalmic artery.
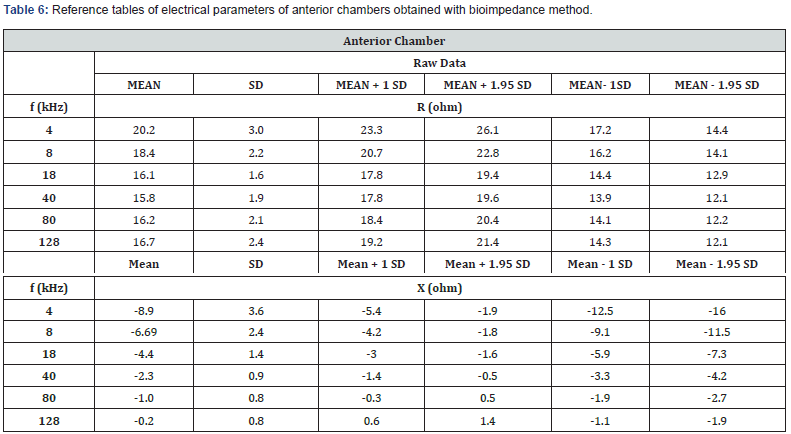
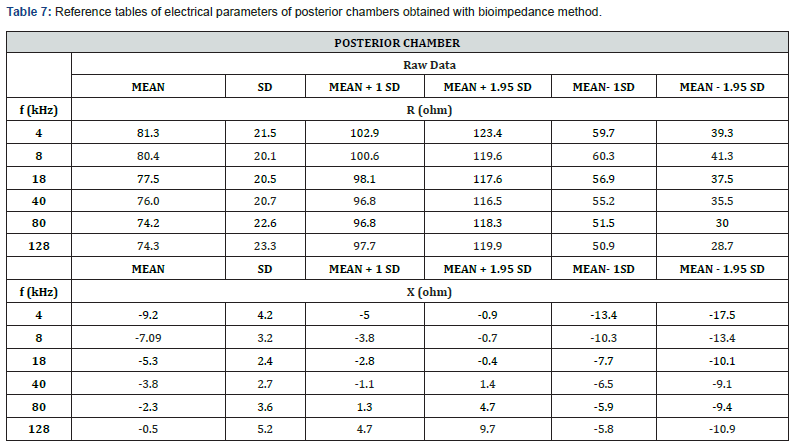
Figure 7 highlights a difference between man (R² = 0.454) and women (R² = 0.638).
Discussion
Firstly, the prototype deserves to be improved to avoid any risk of connection confusion. In addition, currently, we can highlight 3 connectors per measurement line. This must be on-board to minimize the risks of leakage current, parasitic capacitances, and thus improve repeatability at high frequency thus minimizing Hook effect. Finally, the population of this study is still relative and while offering initial results and opening perspectives, does not allow conclusions to be drawn.
Conclusion
This preliminary work has demonstrated the feasibility of this new electrophysiological technique, its ease of use, rapidity, and safety. We obtained a viable repeatability globally less than 1 ohm. We noticed none significatively difference between our estimation of intraocular pressure and estimation of corneal thickness comparatively to the reference (p-value Wilcoxon > 0.1).
This study allowing us to open a new chapter in ophthalmic eye evaluation functions. Further studies need to be projected. To become a clinically useful tool in ophthalmology, and potentially in neuro-ophthalmology, it is necessary to carry out several other complementary studies, for example to determine the influence of age [8,15] on IER (or optic deviations, various pathologies…), and the role of the body’s electrical impedance at the time of measurement. These first results lay encouraging foundations, which with new more developed prototypes and new studies, could allow us to explore new diagnostic tool to explore the fundus of the eye, to measure retinal vascularization and ophthalmic artery, to detect retinal dysfunction, to detect glaucoma with normal pressure, to detect variation in corneal thickness, to detect corneal dysfunction or to detect trabecular meshwork dysfunction.
Acknowledgements
This research project was sponsored by Innosuisse (57541.1 INNO-LS IER) through the InnoCheck subvention plan. This voucher enables PME to commission idea studies and analyses of the innovation and market potential with a Swiss research partner.
We thank gratefully Prof Daniela Nosch and Emanuele Kaeser fellower at FHNW University, Oscar Gris optician-optometrist, Dr Vet Eric Lamouille, Dr Vet Elisabeth Fonteyne.
References
- https://www.draeger.com/fr
- https://www.medis.company
- Jürgens I, Rosellz J, RIu PJ (1996) Electrical impedance tomography of the eye: In vitro measurements of the cornea and the lens. Physiol. Meas 17: A187-A195.
- Grounauer P, Metraux B (2014) The Somnogen Visual Training: A new CBT to fight Insomnia through closed eyes and fNIRS Neuroimagin. J. Behav. Brain Sci 4: 477-481.
- Fukuda M, Sasaki H (2016) In vivo measurement of human corneal impedance value. Cornea J 35(10): 1305-1307.
- Nomura H, Ando F, Niino N, Shimokata H, Miyake Y (2004) The relationship between intraocular pressure and re-fractive error adjusting for age and central corneal thickness. Ophthalmic Physiol Opt 24(1): 41-45.
- Ma Z, Cao P, Sun P, Li L, Lu Y, et al. (2014) Optical imaging of visual cortical responses evoked by transcorneal electrical stimulation with different parameters. Invest Ophthalmol Vis Sci 55: 5320-5331.
- Moreno MV, Coux MN, Clarion A, Grounauer PA (2016) Electrical evolution of aqueous humor with ageing. ICEBI 112: 16.
- Grimnes S, Martisen OG (2006) Wiley Encyclopedia of Biomedical Engineering; John Wiley and Sons: Hoboken. NJ, USA 9.
- Kanai H, Sakamoto K, Haeno M (1983) Electrical measurement of fluid distribution in human legs: Estimation of extra- and intra-cellular fluid volume. J Microw Power 18: 233-243.
- Meijer JH, De Vries PM, Goovaerts HG, Oe PL, Donker AJ, et al. (1989) Measurement of transcellular fluid shift during haemodialysis. Part 1. Method. Med Biol Eng Comput 27: 147-151.
- Bonnet S, Bourgerette A, Gharbi S, Rubeck C, Arkouche W, et al. (2016) Wearable impedance monitoring system for dialysis patients. Annu. Int. Conf. IEEE Eng Med Biol Soc 2016: 5196-5199.
- Moreno MV, Houriet C, Grounauer P-A (2021) Ocular Phantom-Based Feasibility Study of an Early Diagnosis Device for Glaucoma. Sensors 21(2): 579.
- Moreno MV, Grounauer PA (2020) Dispositif Médical D’examen Oculaire. Patent FR2005831.
- Rabbani KS, Sarker M, Akond MHR, Akter T (1999) Focused Impedance Measurement (FIM): A New Technique with Improved Zone Localization. Ann NY Acad Sci 873: 408-420.
- Moreno MV, Coux MN, Clarion A, Grounauer PA (2016) Electrical evolution of aqueous humor with ageing, Book of abstracts, id112, ICEBI p. 16.