Abstract
This paper provides a brief update on recent advances in the reversible free radical recombination. That subject was reviewed in [1]. Here we report the new developments in this area, namely π-stacks of radical-ions and some new systems “radicals-dimer”. The current and potential application of such systems in science and technology is discussed.
Keywords:Recombination; Free radicals; Liquid; Supramolecular; Bond; Hydrocarbons; Complex structure; Triphenylmethyl radicals
Introduction
Many free radicals participate in the reversible recombination:

We will use the notions:

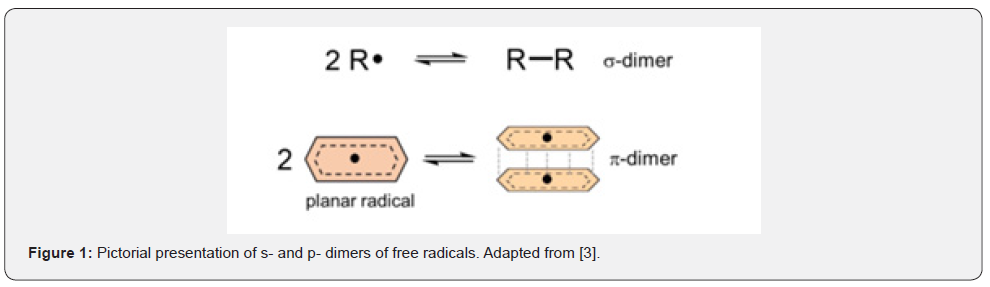
D can be symmetrical or asymmetrical. In most studied cases, the radicals in a dimer are combined by C-C or C-O bonds. Dimers which are formed by a (weak) chemical bond and are called σ- dimers.
The first triphenylmethyl radical, discovered by Gomberg in 1900, took part in the reaction (1) and formed an asymmetrical σ - dimer.
Mainly sterically hindered radicals participate in (1). The delocalization of an unpaired electron over the radical results in its decreased reactivity also. Sterically hindrances prevent the close contact between two .R in D leading to a longer and weaker bond [2].
Results and Discussion
The extensive review article [1] describes many “radicals–dimer” systems with a focus on the kinetics and thermodynamics of reaction (1) and the kinetics of side reactions of .[R]and D besides the reaction (1).
What has happened in that area during the last 45 years?
It was observed that plane radical-ions form π-stacks despite the Coulomb repulsion. They are called π- dimers, Figure 1:
Initially, it was assumed counter-ions are required during dimerization of radical-ions to decrease the Coulomb repulsion [1].
Supramolecular π-dimers can be encapsulated in one or another host [3], which stabilizes dimers [4]. Investigation and reactions of π-dimers in different hosts may lead to the molecular machines [4]. The nature and binding energy of D (reaction (1)) introduces a completely novel recognition motif within supramolecular and mechanically interlocked molecular systems, complementing well-established techniques and enabling new research opportunities [4].
The study of π-dimers leads to understanding and practical use of one type of unusual bonding - the long multi-atom-centered radical dimerization of planar π-systems - within dynamic chemical systems to drive both molecular recognition and mechanical switching [4].
The reversible dimerization of radical-ions of substituted p-quinones and diamines at different temperatures was studied by ESR [5].
There are a few reported examples of when neutral radicals forming π-stacks [4,5].
Usually, the concentration [D] is several orders of magnitude higher than the equilibrium [R]. For example, let the equilibrium [D] = 1.10-3 and the equilibrium [R]= 1.10-7 M.
Consumption of [R] in a side reaction, say Δ [R]= 1.10-7 M, will lead to a negligible change in [D] and will preserve practically the same equilibrium [R]~ 1.10-7 M. Thus, D is a depot of the same quasi-equilibrium [R] for a relatively long time. That should be important for controlled polymerization. Assuming that [R] are reactive towards multiple bonds of a monomer M, and the system (1) is in M solvent or a solution with high [M], we expect that in our example, a low concentration of 1.10-7 M of chain propagating radicals were formed. Slow and constant rates of initiation are required for controlled radical polymerization [6].
2,2’,4,4’,5,5’-Hexaarylbisimidazoles (HABIs) are one of the most widely used thermo- and photo initiators. HABIs is in equilibrium (1) with the corresponding lophyl radicals. HABIs, developed at DuPont, have found applications as initiators in liquid solutions and polymer films [7-9].
Dimers of aromatic 2-arylindane-l,3-dion-2-yl free radicals are efficient inhibitors in liquid-phase oxidation of alkyl aromatic hydrocarbons [10]. Dimers participate in the reaction (1), and the corresponding [R] terminates the oxidation [10].
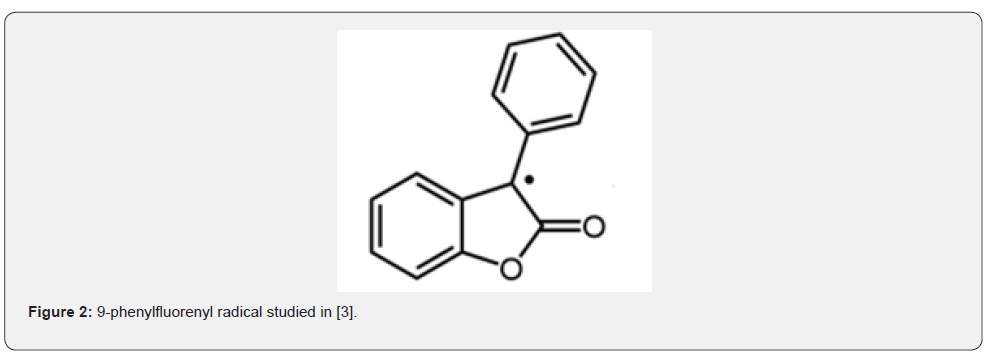
Below are several studied structures of [R] participating in the reaction (1) and leading to s- dimers. 9-Phenylfluorenyl radical. forms a symmetric D Figure 2 [3].
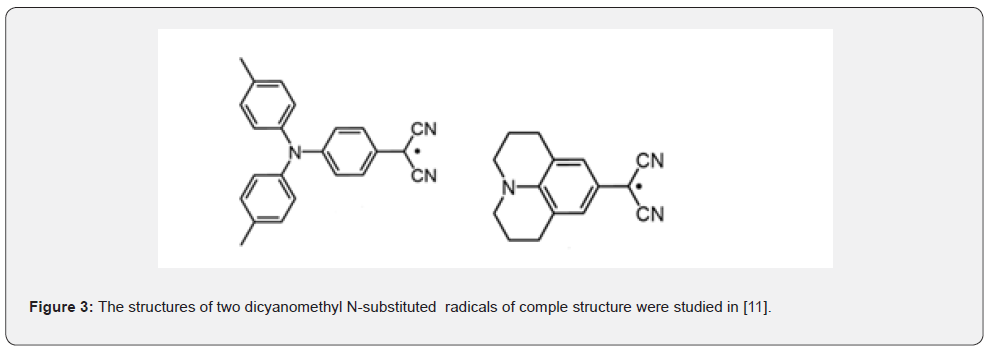
The left radical in the Figure 3 undergoes reaction (1). The right radical with the same reactive fragment is stable, but reversibly dimerizes into p- dimers in a toluene solution [11].
This fact stresses the importance of a large planar structure for the formation of p- dimers.
Studies have shown that para-substituted triphenylmethyl radicals with both electron-donating and electron-withdrawing groups exhibit relatively long lifetimes. It demonstrates lifeincrease of the radicals (lower 2k1) is due to the “captodative” interaction [12].
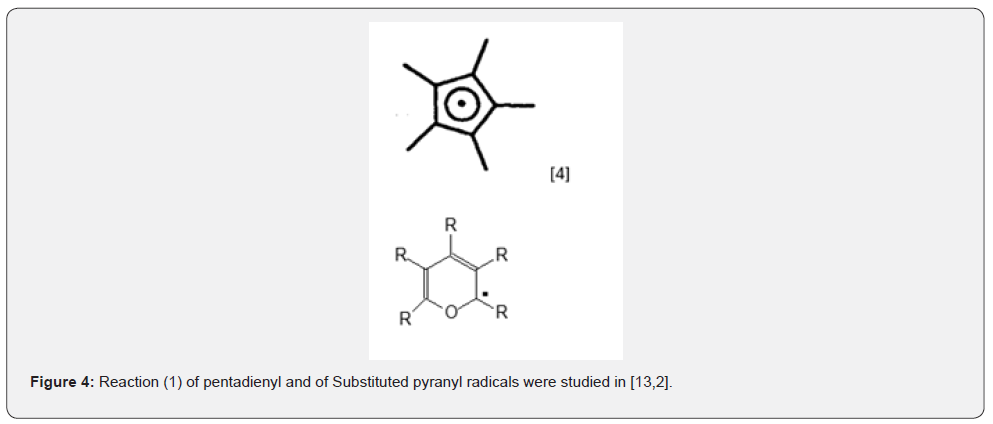
The thermal dissociation of bis(pentamethylcyclopentadienyl) to pentamethylcyclopentadienyl radicals (Figure 4) was studied by EPR spectroscopy [13].
Stable free radicals find an application as photocatalysts [14]. Systems (1) with a sufficient concentration of R. can serve as photocatalysts well.
Conclusion
This area of reversible recombination of radicals (reversible dissociation of dimers) has gradually expanded during the last decades. More such systems were studied by different methods, mainly by ESR and flash photolysis. D are still expected to find a wider application, such as thermo- and photochromic compounds, initiators of controlled polymerization, and antioxidants. D is a convenient source for thermal generation of free radicals [12]. The new supramolecular π -stacks of radical-ions are expected to possess promising properties [2].
The authors of [3] coined the term Dynamic Covalent Chemistry (DCC), i.e., reversible covalent bond formation/scission systems, which is the topic of this paper and of [1]. Molecular self-assembly using the reversible bond formation–dissociation process of organic radicals is a rapidly growing research topic. According to [3], the unique switchable chemical and optoelectronic properties by external stimuli such as mechanical or thermal ones will gain more importance of radical-based DCC as a novel materials platform toward wide-range dyes, sensing, or spintronic applications in the future.
Thus, the reaction (1) known for 125 years attracted much attention in the recent decades. Significant expectations are associated with the study of “dimer-radicals” and especially pdimers [2-4]. Only time will show if they realize.
References
- Khudyakov IV, Levin PP, Kuzmin VA (1980) Reversible Recombination of Radicals. Russ Chem Rev 49(10): 982.
- Antoni PW, Behnke A, Golz C, Hansmann MM (2025) Stabilization of Monomeric Pyranyl Radicals. J Org Chem 90(26): 9108-9117.
- Sakamaki D, Ghosh S, Seki S (2013) Mater. Chem Front.
- Spruell JM (2010) Molecular recognition and switching via radical dimerization. Pure Appl Chem 82(12): 2281-2294.
- Grampp G, Landgraf S, Rasmussen K, Strauss S (2002) Dimerization of organic free radicals in solution. Temperature dependent measurements. Spectrochim Acta A Mol Biomol Spectrosc A 58(6): 1219-1226.
- (1998) Controlled Radical Polymerization, ACS Symposium Series 685, ACS, Washington, DC, Matyjaszewski K, Ed, USA.
- Dessauer R (2006) Photochemistry, History and Commercial Applications of Hexaarylbiimidazoles. Elsevier.
- Caspar JV, Khudyakov IV, Turro NJ, Weed GC (1995) Macromol 28: 636.
- Bendik M, Jedrzejewska B, Paczkiowski J, Linden LA (2002) Hexaarylbisimidazoles and ketocyanine dyes as effective lectron transfer photo initiating systems. Polimery 47(9): 654.
- Pisarenko LM, Nikulin VI, Khudyakov IV (1989) Kinetics of the reversible recombination of substituted 2-(p-dimethlaminophenyl) indane-1,3-dione-2-yl radicals. Bulletin Acad Scie USSR Ser Chem 17: 1544-1545.
- Okino K, Sakamaki D, Hira S, Inoue Y, Seki S (2017) The Divergent Dimerization Behavior of N-Substituted Di cyanomethyl Radicals: Dynamically Stabilized versus Stable Radicals. Angew Chem Intern Edn, 56(52): 16597-16601.
- Neumann WP, Uzick W, Zarkadis AKJ (1986) Sterically hindered free radicals. Substituent-dependent stabilization of para-substituted triphenylmethyl radicals. Am Chem Soc 108(13): 3762-3770.
- Culshaw PN, Walton JC, Hughes L, Ingold KUJ (1993) Dimers of delocalized radicals: pentamethylcyclopentadienyl and pent dienyl. Chem Soc Perkin Trans 2(5): 879-886.
- Khudyakov IV (2023) Electronically Excited States of Free Radicals. Physchem 3(3): 332-341.






























