Abstract
In this contribution we are reporting the mass ratio effect on the active layer of P3HT and PCBM with the electron blocking layer of PANI and PEDOT: PSS organic solar cells. The constructed solar cell system’s performance has been evaluated under a light intensity of 1000W/m². This study used poly (3-hexylthiophene) (P3HT) and [6, 6]-phenyl-C61-butyric acid methyl esters (PCBM) as the active materials. P3HT serves as the donor, whereas PCBM functions as the acceptor. The ratios of PCBM to P3HT are 1:1, 1:2, and 2:1. P3HT: PCBM was combined using toluene solvents. Active layer of P3HT: PCBM of various mass ratios was performed while coating with different spin frequencies from 1000 to 2000rpm. PEDOT: PSS/PANI, the electron blocking layer (EBL), was created for 30 seconds at a constant speed of 2000 rpm. The thin film of PCBM: P3HT has been characterized using UV-Vi’s spectroscopy and J-V characteristics under illumination.
Keywords:Electron blocking layer (EBL); PANI and PEDOT: PSS; Optical band gap; IV- Response; Mass ratio effect
Abbreviations:Voc: Open Circuit Voltage; FF: Fill Factor; HOMO: High Occupied Molecular Orbital; LUMO: Low Unoccupied Molecular Orbital; P3HT: Poly 3-Hexylthiophene; PCBM: Phenyl-C61-Butyric Acid Methyl Ester
Introduction
A variety of organic solar cell architectures are being explored globally, although the donor/acceptor bulk heterojunction polymer solar cell is widely regarded as the most promising method. The fundamental heterojunction comprises a combination of a fullerene-derived electron donor and an acceptor conjugated polymer. When the donor and acceptor have the proper HOMO (High Occupied Molecular Orbital) and LUMO (Low Unoccupied Molecular Orbital) levels, an active material works efficiently [1]. P3HT (poly(3-hexylthiophene)) and PCBM (phenyl-C61-butyric acid methyl ester) exhibit HOMO levels of 3eV and 4eV, respectively, facilitating electron transport from P3HT to PCBM [2]. The polymer semiconductor poly(3-hexylthiophene) or P3HT meets compatibility criteria as a donor material and operates as a p-type semiconductor. The molecular weight of poly(3-hexylthiophene) is 65.5g/mol, and the hole mobility is 3.8-3.9 x 10^-4 cm²/Vs [3]. Poly(3-hexylthiophene) serves as an electron donor, exhibiting a narrow band gap between its LUMO and HOMO, with its maximum absorption wavelength approximately at 650nm.
Conversely, PCBM (6-6-phenyl-C61-butyric acid methyl ether) functions effectively as an electron acceptor. Accepts materials and exhibits strong hole mobility, serving as an electron acceptor in numerous organic devices. PCBM, or Phenyl C61 butyric acid methyl ester, is synthesized from fullerenes. In organic solar cells, PCBM is commonly used as an efficient acceptor material. This makes it possible to compare the PCBM material used in solar cells’ active layer with other materials made from fullerene [4]. Organic polymers possess a broader energy band gap compared to semiconductors. In comparison to other materials, they facilitate effective UV radiation absorption and enhance the wearer’s mobility. A bulk heterojunction structure has been incorporated into many organic photovoltaic materials, specifically P3HT. PCBM mixtures are formulated to extend the donor/acceptor interface [5]. Light incidents on BHJ solar cells are preferentially absorbed by the π-conjugated polymer, resulting in improved coupling accuracy. Literature indicates that the focus can extend to roughly 10nm [6, 7].
When the polymer and fullerene components are separated by an equivalent distance, virtually any excitation can diffuse into the charge transfer area of the fullerene molecule throughout its lifespan, resulting in exciton splitting and the generation of polaron pairs [8,9] quantum performance close to unity quantum performance approaching unity [10,11]. The coulomb-bound charge couples are subsequently separated by a combination of electric potential and concentration gradients [12] and ultimately collected at the electrodes to produce a photocurrent in the external circuit. Numerous research has demonstrated that the optimum shape of the P3HT/PCBM sheet in perovskite solar cells is essential for efficient performance [13,14]. Optimal morphology comprises two primary factors: the configuration of molecules in the donor or acceptor phase, which influences photon absorption and carrier mobility. The extent of donor-acceptor phase separation directly influences exciton dissociation and charge transport and/or collection [15,16] To enhance the power conversion efficiency of inorganic solar cells, it is essential to meticulously examine cell characteristics. A method involves altering the open circuit voltage (Voc), defined as the energy differential between the donor material’s HOMO level and the acceptor material’s LUMO level [17]. Phenyl-C61 butyric acid methyl ester (PCBM) is frequently utilized as an acceptor material in inorganic solar cells. This necessitates structural modification and meticulous calibration of the donor material’s HOMO level. A critical criterion for the efficient functioning of an organic solar cell is a high short-circuit current density (Jsc).
The short-circuit current density of an organic solar cell is significantly influenced by the absorption characteristics of the donor material, which correlates with the material’s band gap [18]. The Jsc can be altered through structural modifications that yield a reduced bandwidth or by adjusting the thickness of the active layer. The fill factor (FF) of an organic solar cell is a crucial parameter influencing cell efficiency, contingent upon Voc, Jsc, and the shape of the active layer [19,20]. The selection of active layer thickness, donor to acceptor ratio, molecular weight of organic materials, electrode composition, and the kind and quantity of organic solvent are critical aspects influencing the performance of bulk heterojunction cells [21]. The PCE of the fabricated solar cell was analyzed for P3HT: PCBM composition ratios of 1:2 and 1:3, and it was concluded that the PCE values were 2.8% [22] and 3.5%, [23] respectively. The equilibrium electron and hole mobility’s in a 1:1 compound was examined and determined to yield an ordered mixture structure [24]. Gold and silver nanoparticles were integrated into the P3HT: PCBM photoactive layer of organic solar cells in varying amounts. The geometry of the active layer, its fabrication method, the optical effects of doping, and the JV analysis of organic solar cells were all examined by including Ag and Au nanoparticles. The findings demonstrate that at an Au NP ratio of 1.5 wt.%, the PCE increased from 2.11% to 3.11%, whereas at an Ag NP ratio of 0.5 wt.%, the PCE increased from 2.11% to 3.20% [25]. Gold and silver nanoparticles in solar cells were engineered to enhance charge transport and augment light scattering [26].
Experimental Section
PEDOT: PSS solution (1 S/cm, 1.3wt.% dispersion in H2O) and undoped Polyaniline (Average Mw=50,000) emeraldine base (EB) was purchased from Sigma-Aldrich.P3HT solution purity 99.995% and PCBM solution Mol. Weight 910.88 was purchased from Otto Chemi pvt ltd. P3HT and PCBM polymers were combined in mass ratios of PCBM to P3HT of 1:1, 1:2, and 2:1. P3HT and PCBM were combined in a toluene solvent. A P3HT: PCBM solution was applied to glass substrates. The deposition was conducted using a spin coater device at varying angular velocities of 1000 to 2000rpm for a duration of 60 seconds [27]. The optical absorbance of the samples was subsequently analyzed using a UV-Vis spectrometer with a wavelength range of 300 to 800nm.
The bulk heterojunction solar cell was fabricated by spin coating on ITO substrates of 1×1cm2, electron blocking layer (PEDOT: PSS/PANI) and Active layer of P3HT: PCBM of different mixture ratio (1:1,1:2,2:1) and different spin frequencies from 1000 to 2000rpm, which were pre-cleaned successively by acetone. After that, layer devices were transferred into vacuum drying oven for annealing at 120∘C for 30 min. Finally, the conducting Ag paste was used to ensure good electrical contact on the surface of active layer and ITO thin films. Current–voltage (I-V) characteristics were recorded by using a Keithley 2400 Electrometer. From the current-voltage curve, the intersecting axis of current and voltage was used to determine the photo sensitizing parameters of open circuit voltage (Voc) and short circuit current density (Isc).
Results and Discussion
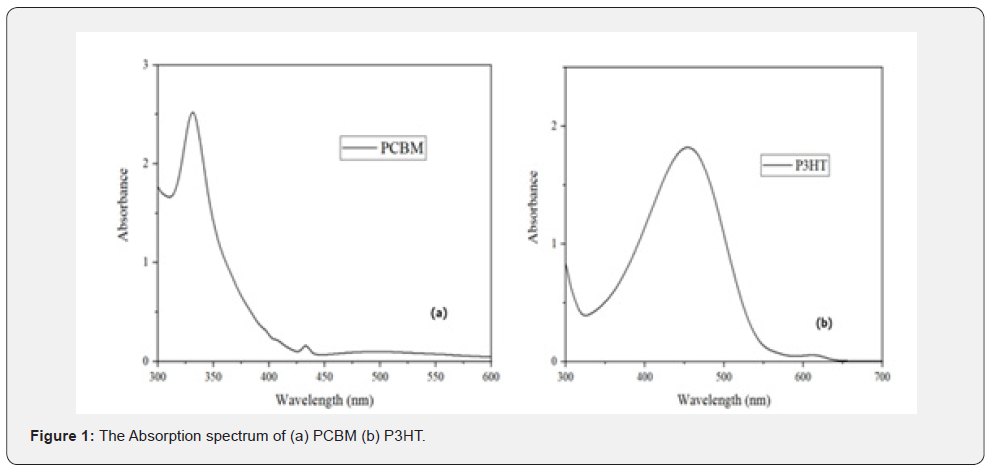
Figure 1 shows the absorption properties of PCBM and P3HT films. The absorption spectra of PCBM demonstrates that the material can absorb light at a wavelength of 330nm, located within the ultraviolet spectrum. P3HT demonstrates absorbance within the 400-500nm region, aligning with the visible light spectrum [28,29].
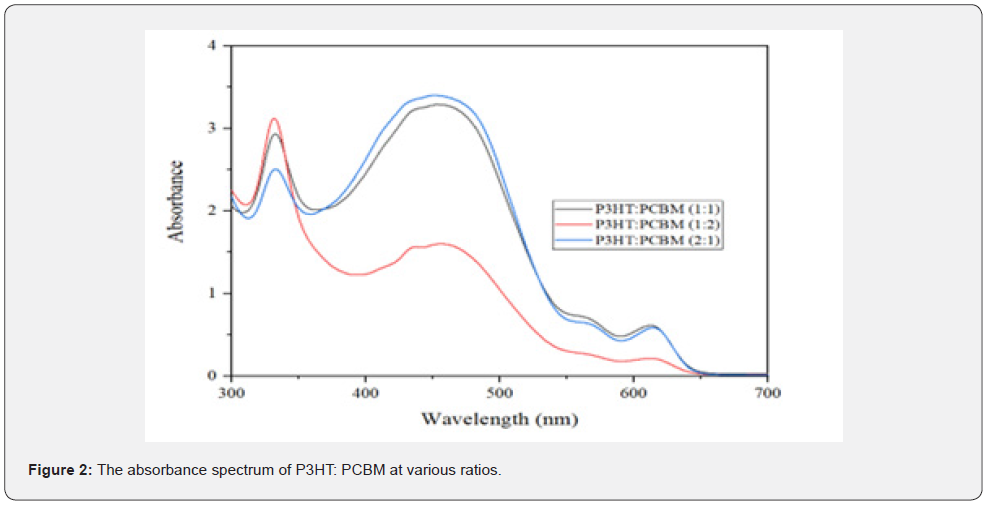
Figure 2 illustrates the absorption characteristics of PCBM and P3HT at different mass ratios (1:1, 1:2, and 2:1). The initial peak occurs inside the absorption spectrum of PCBM, while the subsequent peak is in the absorption spectrum of P3HT. The incorporation of P3HT and PCBM enhances the absorption of the initial and secondary peaks within the visible spectrum. The J-V graph properties of organic solar cells were assessed using a Keithley instrument.
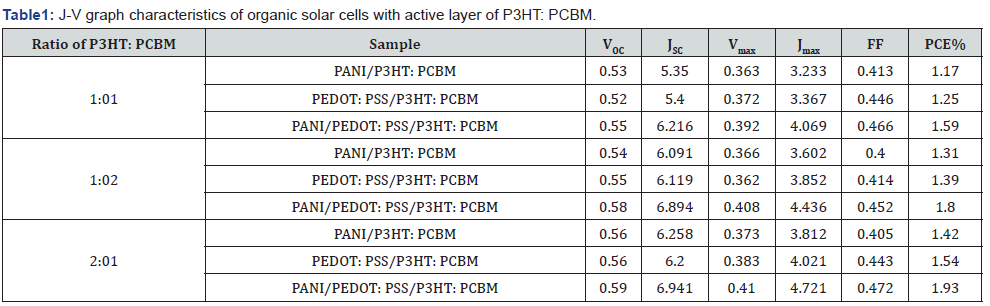
The J-V characteristic graph was utilized to ascertain -Voc, Isc, fill factor (FF), and the efficiency of organic solar cells. Voc is the voltage measured at zero current or under open-circuit conditions. Isc represents the current at zero voltage. The mass ratios of P3HT to PCBM are 1:1, 1:2, and 2:1; consequently, the J-V graph characteristics of organic solar cells exhibit three circumstances. Figure 3 depicts the examination of the J-V properties of organic solar cells.
The ratios of 2:1 and 1:2 yielded high efficiency. Because of its characteristics as an electron donor, Figure 3 illustrates that P3HT can produce more electrons.
Table 1 indicates that the efficiency of organic solar cells improved with the increasing mass ratio of P3HT to PCBM. This is attributable to the capacity of P3HT and PCBM to function within the visible light spectrum, hence generating a greater number of electrons. This absorbance affects the quantity of photons absorbed by organic solar cells. An increase in absorbed photons correlates with a greater generation of electrons. This outcome aligns with the findings of [1] investigation on the donor material.
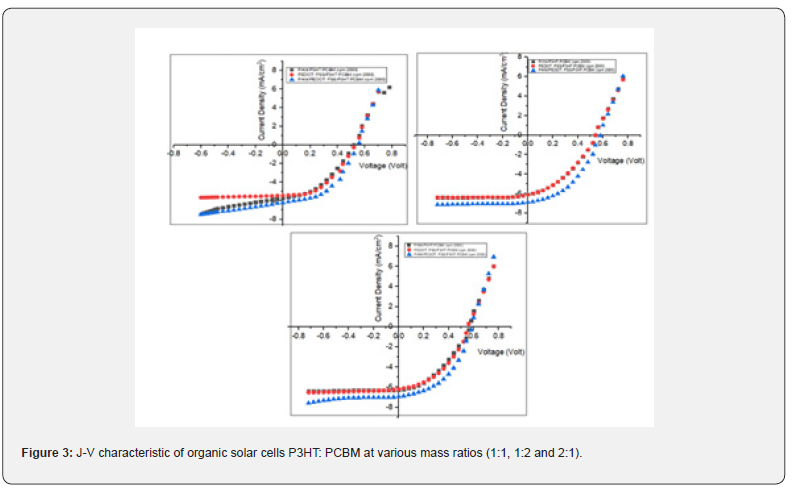
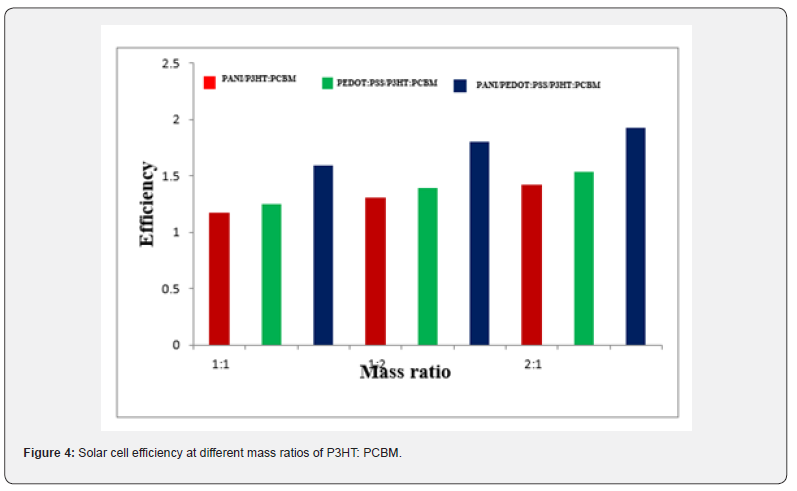
Table 1 shows that the fill factor value increase with an increase in the P3HT: PCBM ratio. A higher fill factor signifies reduced recombination between holes and electrons, as the transport of free charge is functioning effectively [30]. Furthermore, Table 1 presents the characterization of the J-V characteristics of organic solar cells. Table 1 indicates that the efficiency of organic solar cells improved with the increasing mass ratio of P3HT to PCBM. The capacity of P3HT and PCBM to function within the visible light spectrum results in the generation of a substantial number of electrons.
Conclusion
In conclusion, we have methodically examined the correlation between device efficiency and preparation conditions. The photovoltaic properties and charge carrier mobility, particularly hole mobility, of organic solar cells featuring an electron blocking layer of PEDOT: PSS, PANI, and a layer-by-layer structure of PEDOT: PSS and PANI, with varying mass ratios of the active layer composed of P3HT: PCBM, were examined. The optimization of the active layer’s mass ratio enhanced the power conversion efficiency of the organic solar cells from 1.17% to 1.93%. The best efficiency was found to be 1.93% for ITO/PPEDOT: PSS/PANI/ PCBM: P3HT/Ag. The absorption spectrum of PCBM and P3HT material can absorb light in the visible light range. Addition of P3HT and PCBM results in an increase in the absorption of first and second peaks located in the visible region. The ratio 1:2 and 2:1 produced high efficiency can be observed.
Acknowledgement
Authors are thankful to Department of Science and Technology, Govt. of India for financial assistance under DST-FIST scheme (SR/ FST/College-2020-1003 (c)).
References
- Khlyabich PP, Burkhart B, Rudenko AE, Thompson BC (2013) Optimization and simplification of polymer-fullerene solar cells through polymer and active layer design. Polymer 54(20): 5267-5298.
- Supriyanto A, Mustaqim A, Agustin M, Ramelan AH, Suyitno Rosa, et al. (2016) Fabrication of organic solar cells with design blend P3HT: PCBM variation of mass ratio. IOP Conf Ser Mater Sci Eng 107: 012050.
- Kumar A, Li G, Hong Z, Yang Y (2009) High efficiency polymer solar cells with vertically modulated nanoscale morphology. Nanotechnology 20: 165202.
- Supriyanto A, Rosa MES, Iriani Y, Ramelan AH, Nurosyid F (2016) Influences mass concentration of P3HT and PCBM to application of organic solar cells. J Phys Conf Ser 776: 012012.
- Hernández-Como N, Morales-Acevedo (2010) A Simulation of hetero-junction silicon solar cells with AMPS-1D. Sol. Energy Mater Sol Cells 94(1): 62-67.
- Shaw PE, Ruseckas A, Samuel IDW (2008) Exciton Diffusion Measurements in Poly(3‐hexylthiophene). Adv Mater 20(18): 3516-3520.
- Sánchez-Vergara ME, Rios C, Jiménez-Sandoval O, Salcedo R (2020) A Comparative Study of the Semiconductor Behavior of Organic Thin Films: TCNQ-Doped Cobalt Phthalocyanine and Cobalt Octaethylporphyrin. Molecules 25(24): 5800.
- Dai L (1999) Advanced syntheses and microfabrication’s of conjugated polymers, C60-containing polymers and carbon nanotubes for optoelectronic applications. Polym Adv Technol 10(7): 357-420.
- Scharber MC, Sariciftci NS (2013) Efficiency of bulk-heterojunction organic solar cells. Prog. Polym Sci 38(12): 1929-1940.
- Kim Y, Cook S, Kirkpatrick J, Nelson J, Durrant JR, et al. (2007) Effect of the End Group of Regio regular Poly(3-hexylthiophene) Polymers on the Performance of Polymer/Fullerene Solar Cells. J Phys Chem C111(23): 8137-8141.
- Piris J, Dykstra TE, Bakulin AA, Loosdrecht PHM, Knulst W, et al. (2009) Photogeneration and Ultrafast Dynamics of Excitons and Charges in P3HT/PCBM Blends. J Phys Chem C113(32): 14500-14506.
- Gregg BA, Sprague J Peterson MW (1997) Long-Range Singlet Energy Transfer in Perylene Bis(phenethylimide) Films. J Phys Chem B 101(27): 5362-5369.
- Reisdorffer F, Haas O, Rendu PL, Nguyen TP (2012) Co-solvent effects on the morphology of P3HT: PCBM thin films. Synt Met 161(23-24): 2544-2548.
- Konkin A, Bounioux C, Ritter U, Scharff P, Katz EA, et al. (2011) ESR and LESR X-band study of morphology and charge carrier interaction in blended P3HT–SWCNT and P3HT–PCBM–SWCNT solid thin films. Synth Met 161(21-22): 2241-2248.
- Jin SH, Naidu BVK, Jeon HS, Park SM, Park JS et al. (2007) Optimization of process parameters for high-efficiency polymer photovoltaic devices based on P3HT: PCBM system. Sol. Energy Mater Sol Cells 91(13): 1187-1193.
- Jo J, Kim S, Na S, Yu B, Kim D (2009) Time‐Dependent Morphology Evolution by Annealing Processes on Polymer: Fullerene Blend Solar Cells. Adv Funct Mater 19(6): 866-874.
- Brabec CJ, Cravino A, Meissner D, Sariciftci NS, Fromherz T, et al. (2001) Origin of the Open Circuit Voltage of Plastic Solar Cells. Adv Funct Mater 11(5): 374-380.
- Kovacik P, Assender HE, Watt AAR (2013) Morphology control in co-evaporated bulk heterojunction solar cells. Sol Energy Mater Sol Cells 117: 22-28.
- Hazar Apaydın D, Esra Yıldız D, Cirpan A, Toppare L (2013) Optimizing the organic solar cell efficiency: Role of the active layer thickness. Sol. Energy Mater. Sol Cells 113: 100-105.
- Gan WS (2020) Fourier Transform. In: Signal Processing and Image Processing for Acoustical Imaging. Springer, Singapore, pp: 9-11.
- Kocyigit A, Hussaini AA, Yıldırım M, Kose DA, Yıldız DE (2022) Schottky type photodiodes with organic Co‐complex and Cd‐complex interlayers. Appl Organ met Chem 36(11): e6879.
- Padinger F, Rittberger RS, Sariciftci NS (2003) Effects of Postproduction Treatment on Plastic Solar Cells. Adv Funct Mater 13(1): 85-88.
- Schilinsky P, Waldauf C, Brabec CJ (2002) Recombination and loss analysis in polythiophene based bulk heterojunction photodetectors. Appl Phys Lett 81(20): 3885-3887.
- Huang J, Li G, Yang Y (2005) Influence of composition and heat-treatment on the charge transport properties of poly(3-hexylthiophene) and [6,6]-phenyl C61-butyric acid methyl ester blends. Appl Phys Lett 87(11): 112105.
- Liu Q, Jiang Y, Jin K, Qin J, Xu J, et al. (2020) 18% Efficiency organic solar cells. Sci Bull 65(4): 272-275.
- Kaçuş H, Biber M, Aydoğan Ş (2020) Role of the Au and Ag nanoparticles on organic solar cells based on P3HT: PCBM active layer. Appl Phys A 126: 817.
- Gupta ASP, Kumar A, Shree P, Mishra S (2013) Preparation and Characterization of P3ht-Pcbm Organic Solar Cells. Int J Electron Electical Eng 23-25.
- Sun Y, Han Y, Liu J (2013) Controlling PCBM aggregation in P3HT/PCBM film by a selective solvent vapor annealing. Chinese Sci Bull 58: 2767-2774.
- Zuo C, Ding L (2015) Solution-Processed Cu2O and CuO as Hole Transport Materials for Efficient Perovskite Solar Cells. Small 11(41): 5528-5532.
- Agnihotri N (2014) Computational studies of charge transfer in organic solar photovoltaic cells: A review. J Photochem Photobiol C Photochem Rev 18: 18-31.






























