Abstract
The drug delivery system has the function of overcoming biological barriers. Through packaging or coupling, it can resist the instability caused by the microenvironment in the body, increase the targeting of drugs, and reduce toxicity and drug resistance. In other words, by adjusting the delivery and release location of drugs, changing the metabolic behavior of drugs in the body, crossing the physiological barrier to promote drug absorption, to increase drug bioavailability, improve efficacy and reduce toxic side effects. Recently, with the development of pharmacy, biomedicine and other multidisciplinary fields, considerable delivery substances have emerged. This paper reviews the more commonly used delivery systems. For example, GalNAc system, LNP system, exosome system, AAV system, antibody coupling targeting system.
Keywords:Galnac system; LNP system; Exosome system; AAV system; Antibody coupling targeting system
Abbreviations:ASGPR: Asialoglycoprotein Receptors; RISC: Rna-Induced Silencing Complex; DSPC: Di Stearoyl Phosphatidylcholine; APOE: Apolipoprotein E; LDLR: Low Density Lipoprotein Receptor; ADCS: Antibody Conjugated Drugs; PCSK9: Proprotein Convertase Subtilisin/Kexin Type9
Introduction
With the development of pharmacy, more attention has been paid to drug delivery technology. As the emergence of gene therapy, cell therapy and antibody coupling drugs, it is difficult to play a curative effect through traditional technology [1]. The GalNAc delivery system is mainly for small nucleic acid siRNA drugs; LNP delivery system has a wide range of targets, including siRNA, mRNA, gene therapy, etc. Exosome delivery system contains a variety of biological macromolecules, including proteins, nucleic acids, phospholipids, etc. for abundant biological functions, it can be used as a signal of intercellular material communications. AAV system such as hepatotropic AAV8 adeno-associated virus that is described in this paper, which can achieve stable transfection of liver tissue cells and therapeutic expression in liver target tissues by introducing target genes into hepatocytes through intrahepatic or intravenous injection. Antibody coupling targeting system is a class of targeting antibodies combined with traditional cytotoxic small molecule drugs, with good stability in the biological environment outside the target, resulting in the advantages of high specific targeting ability and strong killing effect.
GalNAc Delivery System
N- acetylgalactosamine (GalNAc) conjugates target asialoglycoprotein receptors (ASGPR) in hepatocytes for the delivery of oligonucleotides have become a breakthrough method in the field of therapeutic oligonucleotides. Compared with traditional oligonucleotide delivery methods, the small chemical molecule GalNAc coupled siRNA or ASO technology is a simpler liver delivery method. It shows the advantages of liver targeting specificity, high efficiency, safety and large-scale preparation, which has the research value of further development and broad prospect of clinical applications.
The delivery of GalNAc to the liver is mainly related to the binding of the asialoglycoprotein receptor (ASGPR) on liver cells, where ASGPR expression is much higher than in other parts of the body, showing a very high affinity for GalNAc [2]. Due to the high efficiency and specific gene silencing of RNA interference, GalNAc can effectively combine with siRNA or ASO to enhance plasma stability and effectively deliver it to the liver without inducing innate immune response or hepatotoxicity, which shows great potential for the treatment of liver disease [3]. ASGPR is a receptor that widely existed and specifically expressed on hepatocytes. Human ASGPR contains two variants, ASGPR1 and ASGPR2, which promote the binding of GalNAc into hepatocytes. When pH > 6, ASGPR is encapsulated in membrane vesicles. Acidification during endosome maturation, GalNaC-coupled oligonucleotides are separated from ASGPR, then degraded in lysosomes, furthermore, free ASGRP is further recycled back to the liver cell membrane [4] (Figure 1). The AS strand of the siRNA is formed RNA-induced silencing complex (RISC) with the corresponding mRNA in the cytoplasm and eventually degraded by nuclease. ASO binds to the corresponding precursor mRNA in the nucleus and is then degraded by the RNase H enzyme.
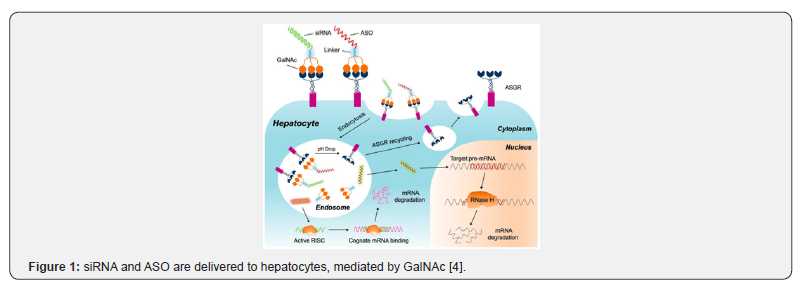
LNP Delivery System
It is known from the literature that LNP is usually composed of four lipid components. Due to the liver targeting, after intravenous injection, LNP is highly enriched in the liver. In 2018, Onpattro, developed by Alnylam, was approved by the U.S. Food and Drug Administration (FDA) to treat multiple neuropathy caused by the hereditary transthyroxin protein amyloidosis, It was the first small interfering ribonucleic acid (siRNA) therapy in history [5,6] Since 2020, due to the outbreak of the COVID-19, LNP has been mainly used for the treatment of mRNA delivery.
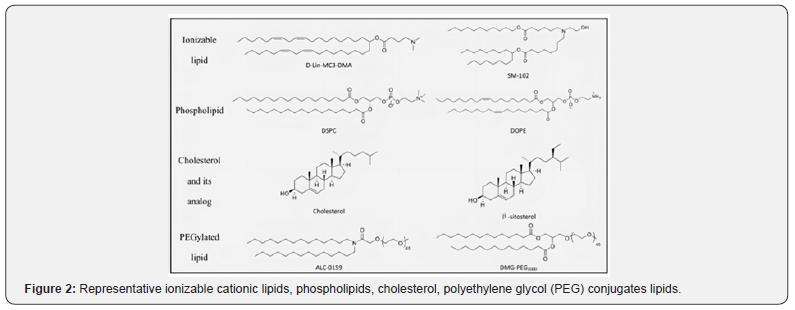
Ionizable lipids, Di stearoyl phosphatidylcholine (DSPC), cholesterol, and PEG-ionized lipids are commonly used in LNP [7] as shown in (Figure 2). Various plasma proteins are absorbed by LNP since entering the blood circulation, to form a “protein crown”. Apolipoprotein E (ApoE) is the key role, that binds to its corresponding liver cell surface receptor (low density lipoprotein receptor (LDLR)), to achieve liver targeted delivery [8]. There will be numerous functional barriers since intravenous injecting into the body, the cell membrane should be penetrated by mRNA to reach the cytoplasm (Figure 3), as it is a dynamic and powerful barrier for intracellular transport. Lipid bilayers are mainly composed of zwitterions and negatively charged phospholipids. The polar head of the phospholipids towards the water environment, and the hydrophobic tail forms a hydrophobic nucleus. These multi-component LNPs are absorbed by endocytosis and electrically bonded to the cell membrane by an inverted non-bilayer lipid phase [9] afterwards wrapped by the lysosome after entering the cytoplasm. Due to the acidity of internal pH, the endosomal membrane is destroyed, resulting in the escape of mRNA, then the naked mRNA is formed in the cytoplasm to normally participate in protein expression.
The encapsulation of mRNA is mainly driven by hydraulic pressure and electrostatic interaction (Figure 4), which is mostly focused on microfluidic hybrid technology, such as T-shaped or Y-shaped microfluidic devices to be processing qualified [10]. There have been a few studies highlighting that four lipids were dissolved in ethanol and other solvents, the mRNA was dissolved in acidic buffer, due to the low solubility of lipids in water and ethanol solvents, they were mixed slowly. After ionization, cationic lipids combined with negatively charged mRNA to form mRNA-LNP, the particle size of which could be controlled by adjusting the fluid injection speed and ratio [11].
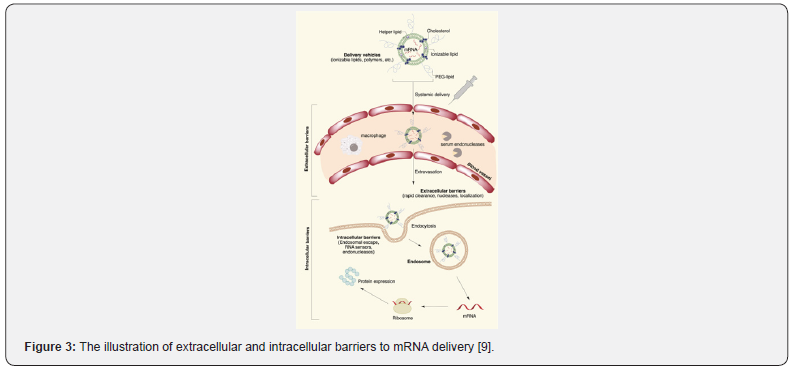
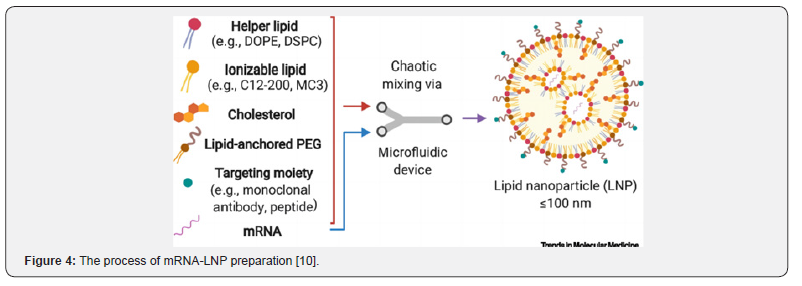
AAV Delivery System
AAV is a small, uncoated linear single-stranded DNA virus, belongs to the genus Dependent virus family of the Parvorder. Its genome is about 4700bp in size and 20-26nm in diameter. As a defective virus, AAV requires helper viruses (adenovirus helper genes include E1a, E1b, E2a, E4orf6 and VA RNA, herpes virus helper genes UL5, UL8, UL22, UL9 and regulatory proteins ICP0, ICP4, ICP22) to infect host cells. Without helper viruses, wild-type AAV can only integrate into the long arm of chromosome19, forming a latent infected and replicated along with host cell chromosome being replicated [12,13] compared with other viral vectors, as low in immunogenicity, AAV does not cause any known human disease, that is expressed primarily in free state, lower integration frequency. AAV has been expanded to new applications, including RNAi and gene editing, further extending the application of AAV vectors.
As a common carrier for gene therapy, the mechanism [14] of AAV is shown in (Figure 5) AAV particles enter the cell through endocytosis by binding to glycosylated receptors expressed on the surface of the host cell, then enter the nucleus through endosomal transporting and escaping. The molting split genome is released, the single-stranded genome is synthesized by complementary double-stranded DNA, transgenic expression or possibly partial integrated into the host genome, transgenic protein biosynthesis - endoplasmic reticulum stress response. Aggregates are formed and variable post-translational modification [15].
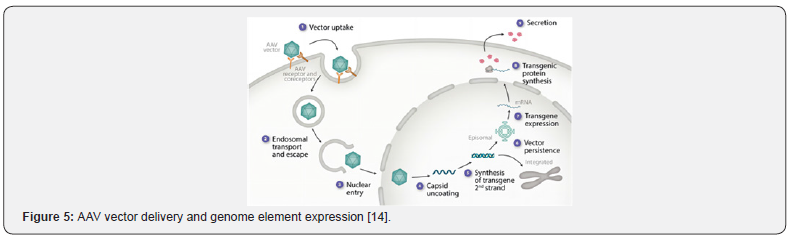
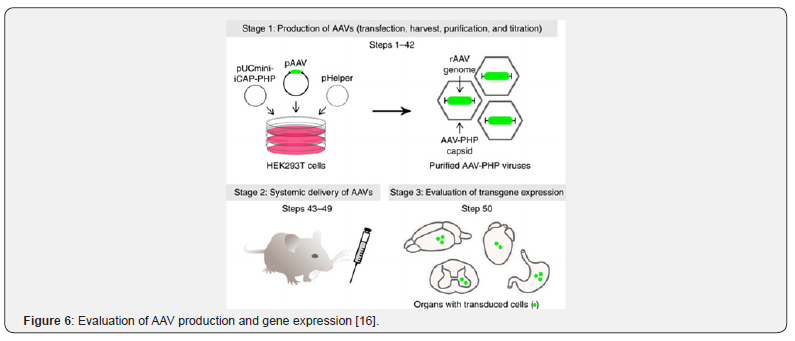
Currently, there are three stages for evaluating in-vivo gene editing vectors (Figure 6), AAV packaging, intravenous injection and transgenic expression were evaluated Firstly, HEK293T cells were transfected with three plasmids: 1) pAAV contains the expressed genome, 2) pUCmini-Icap-PHP encodes viral replication and capsid proteins, 3) pHelper encodes an adenovirus protein necessary for replication. the single-stranded rAAV genome was packaged into the AAV-PHP capsid of HEK293T cells, then AAV-PHP virus was collected and purified, and the titer was determined by quantitative PCR (qPCR). The purified virus was injected into mice through a retroorbital vein. Gene expression was then evaluated by molecular, histological, or functional methods relevant to the experimental target [16].
Exosome Delivery System
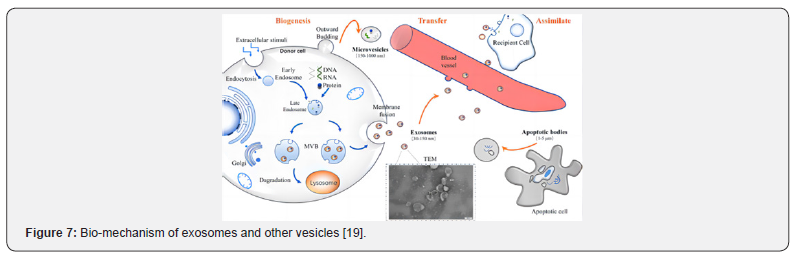
Exosomes are extracellular vesicle structures with biofilm characteristics secreted by body cells, with a particle size of 30- 150nm. All relevant cells in the body can secrete corresponding cell types of exosomes, which have a phospholipid bilayer structure and can carry a variety of important biological information such as proteins, nucleic acids and lipids. For certain factors, the membrane is pitted inward through endocytosis, and the inner membrane formed in this process is called early endosome, that continues to migrate inside the cell (from the extracellular to the nucleus) and gradually mature. As the early endosome matures into the late endosome, the endosome membrane further forms multiple Pitts. Part of the cytoplasm and some substances (such as nucleic acids and proteins in the Golgi apparatus and nucleus) are coated to produce intracavitary vesicles [17], fused with the cell membrane and released into the extracellular environment are defined as exosomes. These exosomes can interact with the extracellular matrix, influence surrounding cells, deliver their contents to target cells through body fluids, even deliver activated receptors and effectors to target cells [18]. The mechanism [19] of exosome delivery to produce biological effects is shown in (Figure 7).
whether therapeutic drugs are directly loaded on exosomes, there are two main types of drug loading: pre-secretory drug loading and post-secretory drug loading. Pre-secretory drug loading means that the therapeutic agent is derived from or loaded on the parental cells to secrete the engineered exosomes. Although this operation is simple, the drug delivery efficiency cannot be controlled, which may destroy the natural physiological function of the membrane proteins. Post-secretory drug loading refers to the direct addition of therapeutic drugs to exosomes in some way, however, there exosome aggregation, membrane damage and low yield may exist. Electroporation methods and ultrasonic methods are the most used drug loading methods, which are relatively convenient and efficient. The drug loading rate of exosome may be related to the hydrophobicity of the drug, the drug loading method and the lipid composition of the exosome. Therefore, the appropriate drug loading method is mainly selected according to the physical or chemical characteristics of the drug [20].
Most current separation techniques are unable to separate exosomes from lipoproteins with similar biophysical properties and extracellular vesicles from non-endosomal pathways, resulting in low purity of exosome. Therefore, how to efficiently enrich exosomes is the main problem at present, and the downstream analysis of exosomes is also very important. For different purposes and applications, different isolation methods have been selected, among which ultracentrifugation, size-based isolation technology, polymer precipitation and immunoaffinity capture technology are commonly used [20,21].
Antibody Coupling Targeting System
Antibody conjugated drugs (ADCs) are usually formed by coupling antibodies to small toxin molecules through ligands, with high targeting of antibodies and high activity of small molecule drugs. They have become a new typical antibody targeted therapy, especially in the field of tumor therapy, with excellent efficacy and potential benefits.
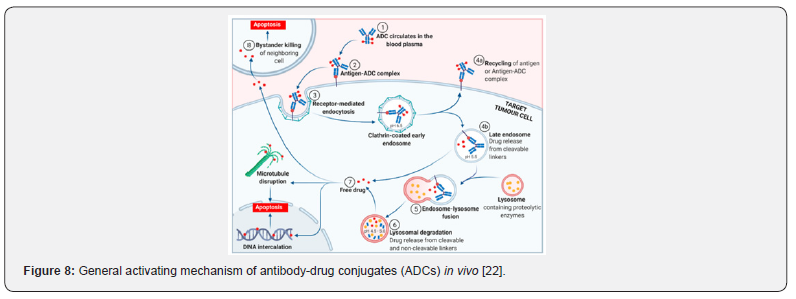
The mechanism [22] of antibody conjugated drugs (ADCs) in vivo is illustrated in (Figure 8). Since ADC enters the plasma circulation, the specific antigens on the surface of tumor cells are recognized and banded to form the antigen-ADC complex. The complex penetrates the cell mainly through receptor-mediated endocytosis, as bond to neonatal Fc receptors, endocytosed in the extracellular space. As the endosome maturing, an environment characterized by pH is changed, those ADCs that remain in the endosome release drugs from the cleavable ligand. The late endosome fuses with the lysosome, in which the ADCs and/or its components are exposed to proteolytic enzymes, promoting further payload release. The free drugs then exert its cell-damaging effects through a pathway specific to the payload mode of action. Most ADC payloads cause apoptosis through DNA damage or tubulin disruption [23,24]. Meanwhile, some payloads (those that are hydrophobic enough to pass through cell membranes) have a bystander effect. The free drugs exit from the target tumor cells, cross the cell membrane and kill neighboring tumor cells, including those that may not express relevant antigens on their cell surfaces or are not easily accessible directly from the circulatory system [25].
For targeted antibodies, the choice of antibody isotypes, IgG1, IgG2, IgG4, has been used to develop therapeutics, but IgG3 isotype has not been used as a therapy due to its significantly faster clearance rate [26]. Moreover, the IgG1 isotype is mostly used in current ADCs. IgG1 isotype may further enhance ADC activity by acting on ADCC (antibody-dependent cell-mediated cytotoxicity) and CDC (complement-dependent cytotoxicity), whereas IgG2 and IgG4 isotypes typically lack their effecting functions. However, IgG4 isotype is selected by PD-1 antibodies, this may be PD-1 antibodies only need to block the interaction between PD-1 and PD-L1 to enhance immune system function, generate anti-tumor activity, and avoid the toxicity of ADCC and CDC on T cells. Therefore, the choice of glycotypes should be carefully taken into consideration. Meanwhile, tumor glycosylated antigen may become the target of ADC if it exists specifically on the surface of tumor cells. The selection of payload mainly depends on the concentration of payload in tumor cells. However, it not only enhances the DAR value of ADCs, but also amplifies the toxicity to normal tissues, with the concentration of payload increased. As a result, the designation of appropriate DAR values should be seriously considered [27,28].
Discussion
For the delivery system of GalNAc and LNP, continuous technological innovation has pushed several products into commercialization and has begun to occupy a dominant position in the market. For instance, after subcutaneous injection, the plasma concentration of GalNAc-siRNA coupling compound is rapidly reduced, for the period of half-life is about 2 to 6h, then rapidly circulates into the liver, the half-life in the liver is about 1 to 6 months. Due to this characteristics, ultra-long-acting administration is achieved, such as lipid-lowering siRNA based on PCSK9 (Proprotein Convertase Subtilisin/Kexin type9) targets, it has been approved for marketing, with advantage of bi-annual dosing, which is much better than traditional small molecule statins and traditional antibody drugs; for the treatment of chronic hepatitis B disease [29] the siRNA delivered into the cytoplasm, the antisense strand combined with mRNA to form a silencing complex, which is degraded by related enzymes, resulting in silencing the expression of related genes. The half-life is much longer than that of traditional small molecule nucleoside analogues (half-life 1 to 3h), and the compliance of patients is significantly improved. However, there are still some disadvantages, no matter if GalNac or LNP, it only targets the liver, and the extrahepatic delivery and targeting efficiency are relatively low. The future will be focused on breakthrough therapies that can be delivered to different tissues and meet the target organ lesions of different tissues.
Although good safety profile and low immunogenicity, the AAV delivery system is divided into a variety of subtype structures that can deliver drugs to different tissues, such as the eye, liver, brain, heart, skeletal muscle, etc. it is confirmed that the transshipment capacity is limited, only 5kb in size. If we want to achieve gene editing, the size of the plasmid packaging genome must be less than 4.5kb, which is obvious challenge at present, scientists are looking for smaller case proteins or modify AAV to improve this behavior [30]. Exosomes are more easily metabolized after delivery, have lower immunogenicity, and can also cross barriers such as the blood-brain barrier that are difficult to pass by other delivery systems.
Antibody-coupled targeting systems, aims at improving the targeting of tumor therapy, reducing toxic side effects, that can target toxins into tumor cells, release toxins and kill specific tumor cells. Up to now, the main problem is that some antibodies have poor selectivity, or the targeted antigen exists in normal tissues, which will cause the toxin to be targeted into normal cells, resulting in targeted toxicity. Secondly, the antibody conjugated drugs have mild decomposed in the blood, a small amount of toxin released, resulting in certain toxicity, that needs to be solved urgently is the main existing problem of ADC drugs at present [31]. We believe that through the elaboration and systematic sorting of the delivery mechanism and characteristics of different drug delivery systems, we can further understand clearly. The goal is to address the shortcomings of each delivery system, and improve drug effectiveness, safety, and compliance.
References
- Garg RKV (2001) Current Status of Drug Delivery Technologies and Future Directions. Pharmaceutical Technology 25(2): 1-14.
- D'souza AA, Devarajan PV (2015) Asialoglycoprotein receptor mediated hepatocyte targeting- Strategies and applications.
J Control Release 203: 126-1a39.
- Wang HX, Xiong MH, Wang YC, Zhu J, Wang J (2013) N-acetyl galactosamine functionalized mixed micellar nanoparticles for targeted delivery of siRNA to liver. J Control Release 166(2): 106-114.
- Cui H, Zhu X, Li S, Wang P, Fang J (2021) Liver-Targeted Delivery of Oligonucleotides with N-Acetylgalactosamine Conjugation. ACS Omega 6(25): 16259-16265.
- Hoy SM (2018) Patisiran: First Global Approval. Drugs 78(15): 1625-1631.
- Akinc A, Maier MA, Manoharan M, Fitzgerald F, Jayaraman M, et al. (2019) The Onpattro story and the clinical translation of nanomedicines containing nucleic acid-based drugs. Nat Nanotechnol 14(12): 1084-1087.
- Hu B, Zhong L, Weng Y, Peng L, Huang Y, et al. (2020) Therapeutic siRNA: state of the art. Signal Transduction and Targeted Therapy 5(1): 101.
- Kowalski PS, Rudra A, Miao L, Anderson DG (2019) Delivering the Messenger: Advances in Technologies for Therapeutic mRNA Delivery. Mol Ther 27(4): 710-728.
- Swingle KL, Hamilton AG, Mitchell MJ (2021) Lipid Nanoparticle-Mediated Delivery of m RNA Therapeutics and Vaccines. Trends Mol Med 27(6): 616-617.
- Guevara ML, Persano F, Persano S (2020) Advances in Lipid Nanoparticles for mRNA-Based Cancer Immunotherapy. Front Chem 8: 589959.
- Na CPZ, LJ L (2022) Progress in research on adeno-associated virus vectors. Chin J Biologicals 35(4): 500-507.
- Samulski RJ, Muzyczka N (2014) AAV-Mediated Gene Therapy for Research and Therapeutic Purposes. Annu Rev Virol 1(1): 427-451.
- Batty P, Lillicrap D (2021) Hemophilia Gene Therapy: Approaching the First Licensed Product. Hemisphere 5(3): e540.
- Li C, Samulski RJ (2020) Engineering adeno-associated virus vectors for gene therapy. Nat Rev Genet 21(4): 255-272.
- Challis RC, Ravindra Kumar S, Chan KY, Challis C, Beadle K, et al. (2019) Systemic AAV vectors for widespread and targeted gene delivery in rodents. Nat Protoc 14(2): 379-414.
- Lin S, Yu Z, Chen D, Wang Z, Miao J, et al. (2019) Progress in Microfluidics‐Based Exosome Separation and Detection Technologies for Diagnostic Applications. Small 16(9): e1903916.
- Pegtel DM, Gould SJ (2019) Exosomes. Annu Rev Biochem 88: 487-514.
- Chen J, Li P, Zhang T, Xu Z, Huang Z, et al. (2022) Review on Strategies and Technologies for Exosome Isolation and Purification. Front Bioeng Biotechnol 9: 811971.
- Zhang Y, Bi J, Huang J, Tang Y, Du S, et al. (2020) Exosome: A Review of Its Classification, Isolation Techniques, Storage, Diagnostic and Targeted Therapy Applications. Int J Nanomedicine 15: 6917-6934.
- Li P, Kaslan M, Lee S H, Yao J, Gao Z (2017) Progress in Exosome Isolation Techniques. Theranostics 7(3): 789-804.
- Tong JTW, Harris PWR, Brimble MA, Kavianinia I (2021) An Insight into FDA Approved Antibody-Drug Conjugates for Cancer Therapy. Molecules 26(19): 5847.
- Jain N, Smith SW, Ghone S, Tomczuk B (2015) Current ADC Linker Chemistry. Pharm Res 32(11): 3526-3540.
- Yaghoubi S, Karimi MH, Lotfinia M, Gharibi T, Birjand MM, et al. (2020) Potential drugs used in the antibody- drug conjugate (ADC) architecture for cancer therapy. J Cell Physiol 235(1): 31-64.
- Goldenberg DM, Sharkey RM (2020) Sacituzumab govitecan, a novel, third-generation, antibody-drug conjugate (ADC) for cancer therapy. Expert Opin Biol Ther 20(8): 871-885.
- Tang H, Liu Y, Yu Z, Sun M, Lin L, et al. (2019) The Analysis of Key Factors Related to ADCs Structural Design. Frontiers in Pharmacology 10: 373.
- Bodyak N, Yurkovetskiy AV (2018) Innovations for Next-Generation Antibody. Drug Conjugates 215-240.
- Tumey LN (2020) Antibody-Drug Conjugates Methods and Protocols. Methods in Molecular Biology
- Yang YC, Yang HC (2022) Recent Progress and Future Prospective in HBV Cure by CRISPR/Cas. Viruses 14(1): 4.
- Chen X, Tan Y, Wang S, Wu X, Yang X, et al. (2021) A CRISPR-Cas12b-Based Platform for Ultrasensitive, Rapid, and Highly Specific Detection of Hepatitis B Virus Genotypes B and C in Clinical Application. Front Bioeng Biotechnol 9: 743322.
- Dan N, Setua S, Kashyap VK, Khan S, Jaggi M, et al. (2018) Antibody-Drug Conjugates for Cancer Therapy: Chemistry to Clinical Implications. Pharmaceuticals (Basel) 11(2): 32.






























