Critical Metals Recovery from E-Waste Printed Circuit Boards using Cyanex 272
Akor Joel Enemona1* and Balogun Ayo Felix1,2*
1Carrs’ Welding Technologies Ltd., UK
Submitted: November 15, 2023; Published: December 18, 2023
*Corresponding author: Balogun Ayo Felix and Akor Joel Enemona, Department of Chemistry, School of Science, Kogi State College of Education (Technical), Nigeria, Department of Industrial, Chemistry, University of Ilorin, Nigeria
How to cite this article: Akor Joel Enemona* and Balogun Ayo Felix*. Critical Metals Recovery from E-Waste Printed Circuit Boards using Cyanex 272. JOJ Material Sci. 2023; 8(2): 555735. DOI:10.19080/JOJMS.2023.08.555735
Abstract
Extracting metals from printed circuit boards (PCBs) in electronic waste (e-waste) is crucial for environmental and economic purposes. E-waste harbors precious metals like copper, gold, silver, and palladium, which can be reclaimed and repurposed. This study investigated the use of bis(2,2,4 trimethylpentyl)phosphinic acid (Cyanex 272) in kerosene for extracting copper and iron from leach liquor obtained from HP E-waste computer printed circuit boards. Influence of various parameters, including the concentration of the extractant, equilibrium pH, and phase ratio, on the degree of copper and iron extraction was examined. Under specific conditions of 25±2 °C temperature, a 1:2 phase ratio, and a 0.2 mol/L Cyanex 272 concentration, extraction efficiencies of 96.8% for copper and 97.1% for iron was achieved within 30 minutes at pH levels of 4.6 and 3.4, respectively. Additionally, about 93.40% of pure copper was successfully stripped from the loaded organic phase at the optimized conditions and hydrometallurgical flow sheet outlining the analytical procedures for the extraction and recovery process was provided (Figure 1).

Keywords: Copper; Iron; Recovery; Cyanex 272; WPCBs; Phase Ratio
Introduction
Printed circuit boards (PCBs) are integral components of electronic and electrical equipment (EEE) and are commonly present in various appliances like fridges, washing machines, TVs, CD/DVD players, personal computers, laptops, mobile phones, modems, radios, and cameras [1-4]. Waste PCBs constitute approximately 3% of the total electronic waste [5]. These discarded PCBs contain diverse elements, including valuable metals such as palladium, gold, silver, and copper, along with polymers, ceramics, and hazardous substances like lead, cadmium, and halogenated flame retardants. Improper disposal of these waste PCBs poses environmental risks and has detrimental effects on the ecosystem [6-9].
Globally, the issue of solid waste pollution has garnered considerable attention [10,11]. With the advancements in electronic technology and improvements in human production and lifestyle, there has been a significant surge in the production and consumption of electronic devices. This has led to a pronounced increase in the disposal of electronic devices, valued at approximately USD 57 billion [12,13], due to their short lifespan. Unfortunately, the majority of discarded electronic equipment cannot be effectively disposed of or reused, primarily due to the lack of mature recycling technologies.
Waste Printed Circuit Boards (WSPCBs) constitute a significant portion of electronic waste. It is projected that, by 2035, WPCBs will consume over 2.31 million tons of copper [14]. Notably, the copper content in WPCBs is several times higher than that found in natural copper ore, where the economic mining value is only 0.5% [15]. Consequently, the recycling of copper from WPCBs serves a dual purpose: it not only reduces dependence on traditional ores but also facilitates the efficient recycling of valuable resources. The extraction of copper from traditional ores has not kept pace with growing demands. As a result, finding alternative sources of copper, such as electrical and electronic wastes, has become crucial. The rapid growth of the electrical and electronic industries in recent decades has led to increased demand for metals like copper. With primary copper resources depleting, production costs rising, and market prices fluctuating, there is a pressing need to develop efficient extraction processes from secondary sources like electronic wastes [7,16-22].
The increasing demand for this metal in industries has resulted in a growing necessity for metal extraction. Solvent extraction, a widely embraced method, is employed to separate and purify copper from leach liquors containing multiple metals. This approach proves highly efficient in recycling Waste Printed Circuit Boards (PCBs) with diverse copper concentrations. Some studies have delved into refining the Pregnant Leach Solution (PLS) of WPCBs through solvent extraction. Oishi et al. [23] utilized LIX 26 to eliminate impurities (Zn, Pb, Mn, Ni, and Fe) from the ammoniaammonium leaching solution of WPCBs, achieving a removal rate of over 95%, thereby facilitating copper recovery. In the domain of precious metals recovery, there are limited studies on WPCBs leaching solution, but various systems, such as organophosphorus derivatives, guanidine derivatives, and amine-organophosphorus derivative mixtures [24], have been explored in other solutions and could potentially prove effective in WPCBs leaching solution. Cui and Zhang [25] conducted a comparison of extractants for aurocyanide complex extraction, revealing that the LIX-79 extractant facilitated gold extraction from alkaline cyanide media, suggesting its potential utility in the cyanide-based leaching solution of WPCBs.
Tanda et al. [26] illustrated the effective utilization of diketones and oxime lixiviants for copper extraction from alkaline glycinate solutions. Their approach allowed for copper extraction, leaving glycinate in the aqueous raffinate post-extraction. In a different investigation, Akbari & Ahmadi [18] demonstrated the efficient two-stage extraction (96%) of copper from bioleaching solution using solvent extraction with LIX 984 N extractant diluted in kerosene. They noted an increased co-extraction of zinc and nickel at higher pH levels (2.5). Wang et al. [27] showcased the industrial viability of M5640 in copper extraction from leach solutions of WWPCBs, achieving over 90.0% copper extraction at pH 1.1, a phase ratio (O/A) of 1/1, M5640 concentration of 16%, and a contact time of 3 minutes at room temperature. In a recent study, Srivastava and Ilyas [28] discovered that thiosulphate facilitated gold stripping from loaded TBP, resulting in 99% recovery efficiency in the solvent extraction process. Furthermore, they achieved successful regeneration of the organic extractant, making it suitable for reuse.
Singh and Rao [29] found that nearly 92% of gold can be reclaimed using optimized parameters through solvent extraction, employing 0.1M tertiary amide for selective and quantitative gold recovery from gold leach solutions. The economic motivation for e-waste recycling lies in the retrieval of precious and base metals, given their substantial value. While past methods prioritized profitable metals such as Cu, Sn, Au, Ag, and Pd, copper stands out as one of the highest-value metal in various electronic scrap materials. Cyanex 272, a phosphine oxide extractant, is widely utilized in the solvent extraction of copper. Its use in this process is advantageous because it forms stable complexes with copper, facilitating efficient extraction and separation. Moreover, Cyanex 272 exhibits selectivity for copper over other metal ions present in the leach solution. Consequently, this study aimed to develop an effective method for extracting copper from the leach liquor of waste printed circuit boards (PCBs) using Cyanex 272. This approach holds promise as an environmentally friendly and economically viable solution for recovering copper and other metals from pregnant solutions of WPCBs.
Materials and Method
Materials
The pregnant leach liquor (PLS) produced from hydrochloric acid leaching was used for the recovery of critical metals. The chemical reagents used in this work were hydrochloric acid (HCl, AnalaR product: 35%), ammonia (NH3, BDH grade), Cyanex 272 (Merck product, purity 85%), and distilled water. Commercial kerosene (diluent) obtained from Olak Filling Station, Ilorin, Kwara State, Nigeria was re-distilled before use. The structure of the extractant is shown in figure 2.

The elemental makeup of the leaching solution was analyzed using Inductively Coupled Plasma-Mass Spectrometry (ICPMS: Agilent HP 7700 ICP-MS model) and an atomic absorption spectrophotometer (AAS: BUCK Scientific ACCUSYS 211 Atomic Absorption Spectrophotometer), as detailed in table 1.

Solvent extraction
The extraction and stripping tests were carried out within a sealed container using a mechanical agitator equipped with glass blades to facilitate thorough mixing. Precise temperature control was maintained using a water bath. The equilibrium pH was adjusted by the direct addition of either concentrated HCl solution (10% volume fraction) or NH3 solution (1 mol/L). Initial experiments, as reported by Correa et al. [30], indicated that the equilibrium between the two phases could be rapidly established. For the solvent extraction studies, Cyanex 272 was dissolved in distilled diluent. Batch experiments were conducted at room temperature (27 ± 2 °C) by mixing equal volumes (25 ml) of Cyanex 272 in kerosene diluent with leach liquor having a pH of 0.69. This mixture was agitated for 30 minutes using a Gallenkemp thermostated orbital shaker [18,27,31]. After equilibration and phase separation with a separating funnel, the copper content in the aqueous sample was determined using ICP-MS and AAS. The critical metal content in the organic phase was calculated using a mass balance. To understand the copper extraction behavior, this study delved into the impacts of extractant concentration, solution pH, and phase ratio on the degree of metal extraction.
Results and Discussion
Solvent Extraction Studies
The pregnant leach liquor (PLS) produced through hydrochloric acid leaching served as the medium for critical metal recovery. Under optimized conditions, the composition of the acid PLS was determined to be 285.1 mg/L Cu, 102.7 mg/L Al, 224.3 mg/L Fe, 12.07 mg/L Pb, and 11.9 mg/L Mn. This leach liquor was then employed for the selective separation of copper from other metals.
Effect of Cyanex 272 concentration
In this investigation, the influence of varying the concentration of the extractant, Cyanex 272, in the range of 0.01 mol/L to 0.3 mol/L at 25 ± 2 ºC for 30 minutes on the extraction of copper, aluminum, iron, lead, and manganese from HP WPCBs’ aqueous solution was examined. The findings, illustrated in figure 3, demonstrated that the order of metal ion extraction was Fe > Cu > Pb > Mn > Al, consistent with prior research [30,32]. This implies the potential for selectively separating iron, copper, and lead from other elements [31]. The study noted that higher extractant concentrations correlated with increased metal extraction. For example, copper extraction increased from 20.4% at 0.01 mol/L to 61.9% at 0.2 mol/L. Similar trends were observed for other metals, except for manganese and aluminum, present in low to trace amounts. Consequently, only metals present in significant proportions were considered for further analysis.
To determine the quantity of Cyanex 272 involved in the extraction process, the impact of Cyanex 272 on the distribution ratio (D) of key metal ions (Cu, Fe, Al) was investigated, as shown in figure 4. The relationship between log D and log [Cyanex 272] was graphed (Figure 4). The plots yielded straight lines with a slope of around 1 for all the metals examined, indicating the association of one mole of Cyanex 272 extractant with the extracted metal species. This analysis helped assess the extent of Cyanex 272’s involvement in the extraction process.


Effect of pH on metals extraction
The influence of pH on the extraction of metals was assessed using a chloride leach solution. The initial pH was systematically varied within the range of 1.0 to 5.0 at a temperature of 25 ± 2 ºC. The results, illustrating the percentage of metal ion extraction for copper, iron, and aluminum, were graphically represented against the equilibrium pH, as depicted in figure 5.
Figure 5 illustrates that the extraction percentages increased with higher equilibrium pH levels, except for aluminum, which exhibited a decrease with rising pH. The optimal extraction occurred at equilibrium pH values of 4.6, 3.4, and 1 for copper, iron, and aluminum respectively. Banda et al. [33] confirmed in a similar study that solvent extraction of Mo and Co from chloride solution containing Al was effective for Co and Mo, while extraction of Al was negligible. Consequently, it can be deduced that Cyanex 272 is not suitable for separating Al from aqueous chloride solution containing Cu and Fe, possibly due to its preference for extracting HCl over Al [33]. Additionally, Cyanex 272 might only be capable of extracting anionic species of aluminum from aqueous chlorides. Figure 6 demonstrates the effect of equilibrium pH on log D, indicating the involvement of hydrogen ions in the extraction process. The plot showed a linear relationship for all metal ions, suggesting the liberation of one mole of H+ during extraction in each case, with slopes of approximately 0.932, 0.763, and 0.847 for copper, iron, and aluminum respectively [34].


Effect of Phase ratio on Cu and Fe extraction
In this investigation, the extraction of copper and iron from a chloride solution was scrutinized at different A/O (aqueous/ organic) ratios - specifically, 1:1, 1:2, and 2:1 - while maintaining equilibrium pH levels at 4.6 for copper and 3.4 for iron. The results, summarized in table 2, indicated that a higher concentration of extractant in the organic phase, particularly at the ratio of 1:2, fostered increased interaction between extractant molecules and copper/iron, leading to elevated recovery rates. For instance, at an A/O ratio of 1:2, the maximum extractions reached 96.8% for copper and 97.1% for iron at pH levels of 4.6 and 3.4, respectively.

Stripping of metal ions from the loaded organic phase
The process of stripping copper and iron from the loaded Cyanex 272 involved the use of various concentrations of HCl solutions: 0.01 mol/L, 0.05 mol/L, 0.10 mol/L, 0.20 mol/L, and 0.50 mol/L. The outcomes of these experiments are detailed in table 3. The results indicated that complete removal of copper was achieved with 0.1 mol/L HCl solutions in a single contact at a phase ratio of A/O 1:2.

Operational metals recovery flow sheets
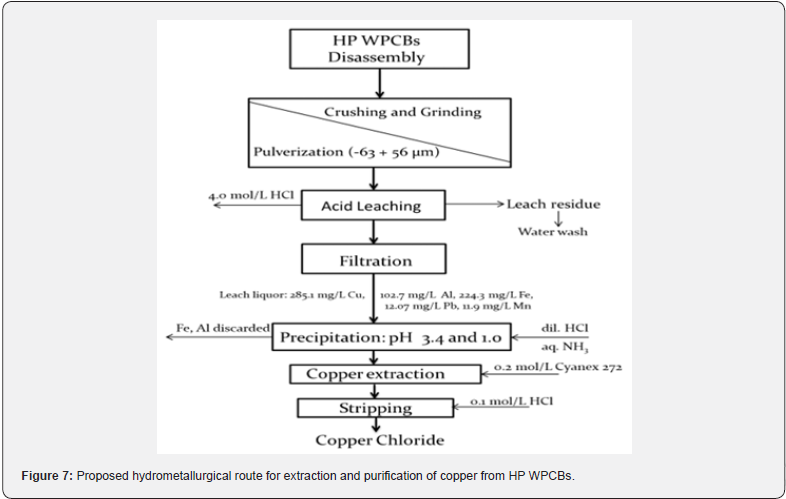
Figure 7 illustrates a hydrometallurgical scheme that summarizes the analytical methods used to treat E-waste HP PCBs, aiming to separate copper from other metal ions.
Conclusion
This study explored the extraction of copper and iron from hydrochloric acid-leached liquor using bis(2,2,4-trimethylpentyl) phosphinic acid (Cyanex 272) in kerosene. The findings revealed that quantitative stripping was accomplished with 0.1 mol/L HCl solutions, whereby approximately 93.4% of copper from the loaded organic phase was stripped at a phase ratio of A/O 1:2. A comprehensive hydrometallurgical flowchart outlining the analytical procedures for extracting and purifying copper leach liquor to obtain copper chloride from leached liquor of HP E-waste computer printed circuit boards was developed.
Acknowledgement
The researchers are thankful to Tertiary Education Trust Fund (Tetfund) Nigeria for providing financial support.
References
- Cabrera JM, Canal A, Cabrera J, Fraga CD (2013) Printed circuit boards: a review on the perspective of sustainability. J Environ Manage 131: 298-306.
- Li J, Lu H, Guo J, Xu Z, Zhou Y (2007) Recycle technology for recovering resources and products from waste printed circuit boards. Environ Sci Technol 41: 1995-2000.
- Verma HR, Singh KK, Mankhand TR (2017a) Delamination mechanism study of large size waste printed circuit boards by using dimethylacetamide. Waste Manag 65: 139-146.
- Widmer R, Oswald KH, Sinha KD, Schnellmann M, Böni H (2005) Global perspectives on e-waste. Environ Impact Assess Rev 25: 436-458.
- Bizzo WA, Figueiredo RA, Andrade VFD (2014) Characterization of Printed Circuit Boards for Metal and Energy Recovery after Milling and Mechanical Separation. 7(6): 4555-4566.
- Szałatkiewicz J (2014) Metals content in printed circuit board waste. Pol J Environ Stud 23(6): 2365-2369.
- Kumari A, Jha MK, Lee JC, Singh RP (2016b) Clean process for recovery of metals and recycling of acid from the leach liquor of PCBs. J Clean Prod 112(5): 4826-4834.
- Verma HR, Singh KK, Mankhand TR (2016) Dissolution and separation of brominated epoxy resin of waste printed circuit boards by using dimethyl formamide. J Clean Prod 139: 586-596.
- Xiu FR, Qi Y, Zhang FS (2015) Leaching of Au, Ag, and Pd from waste printed circuit boards of mobile phone by iodide lixiviant after supercritical water pre-treatment. Waste Manag 41: 134-141.
- Arub S, Ahmad S, Ashraf S, Majid Z, Rahat S, et al. (2020) Assessment of waste generation rate in teaching hospitals of metropolitan city of Pakistan. J Civ Eng 6(9): 1809-1821.
- Buaisha M, Balku S, Yaman S (2020) Heavy metal removal investigation in conventional activated sludge systems. J Civ Eng 6(3): 470-477.
- Li H, Oraby E, Eksteen J (2021) Cyanide consumption minimization and concomitant toxic effluent minimization during precious metals extraction from waste printed circuit boards. Waste Manag 125: 87-97.
- Akcil A, Erust C, Gahan CS, Ozgun M, Sahin M, et al. (2015) Precious metal recovery from waste printed circuit boards using cyanide and non-cyanide lixiviants a review. Waste Manag 45: 258-271.
- Zhang S, Gu Y, Tang A, Li B, Li B, et al. (2020) Forecast of future yield for printed circuit board resin waste generated from major household electrical and electronic equipment in China. J Clean Prod 283: 124575.
- Cao J, Chen Y, Shi B, Lu B, Zhang X, et al. (2016) WEEE recycling in Zhejiang Province, China: generation, treatment, and public awareness. J Clean Prod 127: 311-324.
- Vijayaram RR, Nesakumar D, Chandramohan K (2014) Copper Extraction from the Discarded Printed Circuit Board by Leaching. Int J Chem PetrochemTech (IJCPT) 4(4): 11-16.
- Eksteen J, Oraby E, Li H (2018) Hydrometallurgical recovery of metals from waste printed circuit boards (PCBs): Current status and perspectives - A review. Resources, Conservation & Recycling 139: 122-139.
- Akbari S, Ahmadi A (2019) Recovery of copper from a mixture of printed circuit boards (PCBs) and sulphidic tailings using bioleaching and solvent extraction processes. Chemical Engineering and Processing - Process Intensification 142: 107584.
- Khojiev ST, Ergasheva MS, Khamroqulov SF, Khamroev JO (2021) The Current State of Copper Metallurgy and Its Raw Material Base. International Journal of Engineering and Information Systems (IJEAIS) 5(5): 7-14.
- Mudd GM, Weng Z (2012) Base Metals. In: Letcher TM, Scott JL (eds.), Materials for a Sustainable Future. London: Royal Society Chem p. 11-59.
- Mudd G, Weng Z, Jowitt SM (2013) A detailed assessment of global Cu reserve and resource trends and worldwide Cu endowments. Economic Geology 108(5): 1163-1183.
- Northey S, Mohr S, Mudd GM, Weng Z, Giurco D (2014) Modeling future copper ore grade decline based on a detailed assessment of copper resources and mining, Resources, Conservation and Recycling 83: 190-201.
- Oishi T, Koyama K, Alam S, Tanaka M, Lee JC (2007) Recovery of high purity copper cathode from printed circuit boards using ammoniacal sulfate or chloride solutions. Hydrometallurgy 89(1): 82-88.
- Wu Z, Yuan W, Li J, Wang X, Liu L, et al. (2017) A critical review on the recycling of copper and precious metals from waste printed circuit boards using hydrometallurgy. Front Environ Sci Eng China 11(5): 8.
- Cui J, Zhang L (2008) Metallurgical recovery of metals from electronic waste: a review. J Hazard Mater 158: 228-256.
- Tanda BC, Oraby EA, Eksteen JJ (2017) Recovery of copper from alkaline glycine leach solution using solvent extraction. Sep Purif Technol 187: 389-396.
- Wang L, Sun X, Wang L (2018) Separation and recovery of copper from waste printed circuit boards leach solution using solvent extraction with Acorga M5640 as extractant, Separation Sci Tech 54(8): 1-10.
- Srivastava RR, Ilyas N (2023) Solvent extraction of gold from a chloride-hypochlorite leached solution of waste printed circuit boards. Geosystem Eng 26(5): 190-199.
- Singh KK, Rao MD (2023) Recycling of Copper and Gold from Waste Printed Circuit Boards by Leaching Followed by Solvent Extraction. In: Ouchi T, et al. Rare Metal Technology. TMS 2023. The Minerals, Metals & Materials Series, Springer, Cham.
- Correa F, Silvas PS, Aliprandini P, Moraes VT, Dreisinger D, et al. (2018) Separation of Copper from a Leaching Solution of Printed Circuit Boards by Using Solvent Extraction with D2EHPA. Brazilian Journal of Chemical Engineering 35(3): 919-930.
- Abhilash TS, Ghosh A, Meshram P, Van Hullebusch ED (2021) Microbial Processing of Waste Shredded PCBs for Copper Extraction Cum Separation - Comparing the Efficacy of Bacterial and Fungal Leaching Kinetics and Yields. Metals 11: 317.
- Sole KC, Hiskey JB (1992) Solvent extraction characteristics of thiosubstituted organophosphinic acid extractants. Hydrometallurgy 30(1-3): 345-365.
- Banda R, Hosohn S, Seung LM (2013) Solvent extraction separation of Mo and Co from chloride solution containing Al. Materials Transition 54: 61-65.
- Kumar P, Panda R, Kumar M, Lee J, Pathak DD (2016a) Recovery of copper and recycling of acid from the leach liquor of discarded Printed Circuit Boards (PCBs). Sep Purif Technol 156(2): 269-275.






























