Mineral Binding Material using Red Mud
Lev Chernyak* and Natalia Dorogan
National Technical University of Ukraine, Igor Sikorsky Kyiv Polytechnic Institute, Kyiv, Ukraine
Submitted: September 15, 2023; Published: October 04, 2023
*Corresponding author: Lev Chernyak, National Technical University of Ukraine, Igor Sikorsky Kyiv Polytechnic Institute, Kyiv, Ukraine Email: Ipchernyak@ukr.net
How to cite this article: Lev Chernyak* and Natalia Dorogan. Mineral Binding Material using Red Mud. JOJ Material Sci. 2023; 8(2): 555731. DOI:10.19080/JOJMS.2023.08.555731
Abstract
The possibility of producing a mineral binder material for low-temperature firing (≤ 1200°C) with the integrated use of natural raw materials and alumina production waste - red mud has been studied. An analysis was carried out of the dependence of the binary and ternary compositions of the initial raw material mixtures on the specified characteristics of the binder material using the developed computer program “Roman Cem”. The compositions of mixtures for the manufacture of mineral binder material using 2.4-29.1 wt. % of red mud as technogenic raw material was determined. The features of the formation of the phase composition and properties of the binder during firing of raw material mixtures with a maximum temperature of 1100oC are shown.
Keywords: Material; Mineral binder; Red mud; Raw material mixture; Composition; Firing; Phases crystalline; Properties
Introduction
The technology of mineral binding materials is associated with the use of large volumes of carbonate and clay natural raw materials, the resources of which are exhaustive and non-renewable [1-3]. The modern requirements of resource saving [4-8] correspond to the reduction of the use of natural raw materials with the replacement of them by man-made materials - waste from various industries [7-11].
In the production of cement, the use of waste from other industries is known and regulated by current standards [12,13], mainly as substitutes for part of the clinker when it is finely ground. At the same time, granulated blast furnace slag and TPP fly ash are used in the largest quantities [14,15]. A small amount (1.5 - 5.0 wt.%) of iron-containing industrial waste is introduced as fluxing additives to the composition of the raw material mixtures for the production of clinker, which slightly affects the volume of their utilization. It is obvious that the increase in the content of man-made components in the composition of mass-intensive starting mixtures for the production of clinker will contribute to the comprehensive solution of the problems of resource saving and provision of the raw material base of industrial production.
The practical solution of such problems requires determining the patterns of influence of raw materials of different genesis and composition on the structure formation and properties of products, in the direction of which research and development are carried out. At the same time, multi-ton wastes of ferrous and non-ferrous metallurgy attract attention as a possible man-made raw material.
Wastes of non-ferrous metallurgy [16] are slags, sludges, ore beneficiation wastes. Thus, a by-product of the processing of bauxite into alumina according to Bayer’s method is red mud [17-19]. According to the specified industrial method, during the treatment of bauxite with caustic soda, approximately 35-40% of the original ore goes to waste, forming an alkaline red mud with a concentration of the solid phase of 15-40%. As a result, 0.8-1.5 t of red mud is formed during the production of 1 ton of alumina. Data are available that with the annual world production of 101 million tons of alumina, 120 million tons of red mud are being produced, including about 1 million tons in Ukraine during the operation of Mykolayiv Alumina Plant and Zaporizhzhya Aluminum Combine.
Red mud is characterized by an increased content of iron oxides, ferrites and calcium alumina ferrites, aluminates, aluminosilicates and sodium ferrites [20]. Large volumes of accumulation of red mud create an ecological hazard, which emphasizes the relevance of the development of its disposal, if to take into account physical and chemical properties and the impact on the characteristics of silicate systems and the properties of the final product [21-23]. From the point of view of the volumes of formation and composition, the use of red mud as a man-made raw material in the mass production of mineral binding materials is promising [24-26]. At the same time, the choice of the most acceptable technical solutions for a significant increase in the amount of man-made raw materials should be based on the development and implementation of new compositions based on relevant research of silicate systems, the results of which are presented in this work.
Experimental Part
The object of the study was raw material mixtures for the production of low-temperature fired cement with the complex use of natural and man-made raw materials. The raw mixtures were prepared by dosing the components by weight, mixing and homogenizing in a ball mill, firing and grinding the final product according to modern cement technology.
Samples of raw mixtures were fired in a furnace for 15 hours at a maximum temperature of 1050-1400°C, keeping at a maximum of 1.5 hours. All samples of the mixtures that were compared were fired at the same time to exclude the possibility of a difference in the degree of heat treatment. The methods of physical-chemical analysis of silicate raw materials and testing of binder properties used in this work included:
a) analysis of chemical composition using standardized procedures.
b) X-ray diffraction analysis (powdered drugs) using diffractometers DRON-4-0 and Philips X’Pert PRO - MRD, connected through an interface to a computer.
c) determination of cement properties in accordance with current standards.
Various types of raw materials were used to determine the rational composition of the initial mixture:
a) chalk of Zdolbuniv deposit in Rivne region and limestone of Dubovetsky deposit in Ivano-Frankivsky region
b) marl of Dubovetsky deposit in Ivano-Frankivsky region (marl D) and marl of Bakhchisaraysky deposit in Crimea region (marl B)
c) pyloquatz of PJSC “Novoselitsky mining and beneficiation plant” of Kharkiv region
d) perlite from the Berehiv deposit of Zakarpattia region
e) red mud - alumina production waste of PJSC Mykolaiv Alumina Plant (MGZ) and PJSC Zaporizhzhya Aluminum Plant (ZALK).
Samples of raw materials differ significantly in their genesis and composition. According to the chemical composition, among the studied raw materials, samples of Zdolbniv chalk and Dubovets limestone, known for their use in cement production, are characterized by a high content of CaO (Table 1).
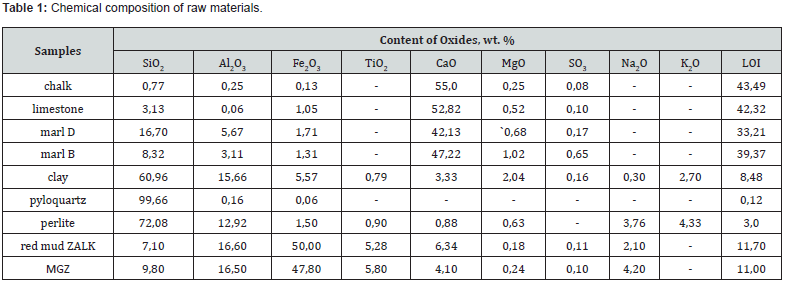
Marl samples differ from chalk and limestone in a significantly higher content of silicon and aluminum oxides, and in their content and quantitative ratio of SiO2: Al2O3. The sawdust sample is characterized by a predominant silica content, the pearlite sample by a significant amount of silica with a ratio of SiO2: Al2O3 = 6 : 1 and alkaline oxides of the type R2O = 8.09 wt. %. Red mud samples are characterized by a high content of iron oxides and have differences related to the composition of the raw bauxite raw material and technological parameters of its processing. Thus, the MGZ sample is characterized by a slightly higher content of SiO2, TiO2 and Na2O than that of ZALK with a slightly lower amount of CaO.
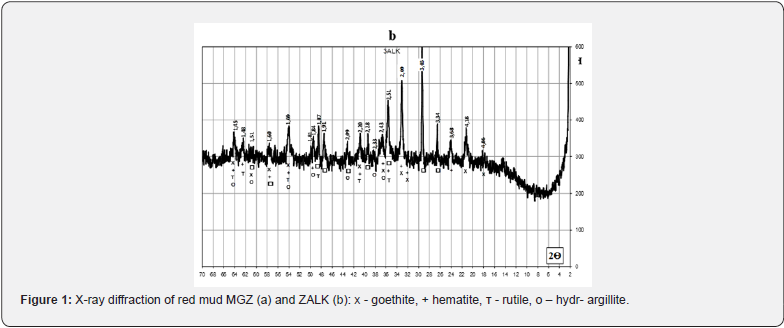
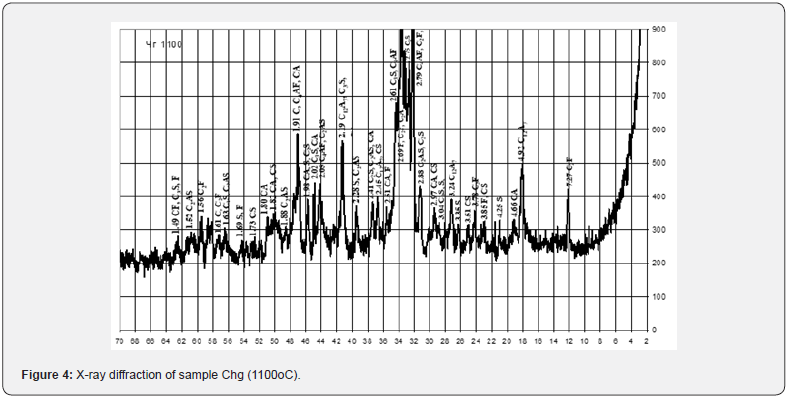
The main rock-forming mineral of chalk and limestone is calcite with admixtures of dolomite, quartz and kaolinite. Marl samples are a natural mixture of carbonate and clay rocks and, accordingly, the rock-forming minerals calcite, kaolinite, and quartz. Sawdust consists mainly of crystalline quartz, the pearlite sample is characterized by a developed glass phase with crystalline inclusions of quartz and mullite. The mineralogical composition of red mud samples is characterized by the presence of goethite Fe2O3∙H2O, hematite Fe2O3, hydrargillite Al2O3∙3H2O, rutile TiO2 and ilmenite FeTiO3 (Figure 1). In order to identify the possibility of increasing the amount of red mud utilization in the technology of binding materials, an analysis of the composition of raw materials for the production of material of the natural or Roman cement type was carried out. The composition of the raw material mixtures was determined in accordance with known recommendations for Roman cement technology in the range of specified values of the hydraulic modulus НМ=1.1 - 1.7 using computer calculations using «RomanCem» program [27].
The analysis of the obtained results showed that in the specified HM interval, the possible concentration of both studied samples of red mud in the composition of binary raw mixtures significantly depends on the types of carbonate components (Figure 2), while there is an inversely proportional relationship between the content of the mud and the number of the hydraulic module.
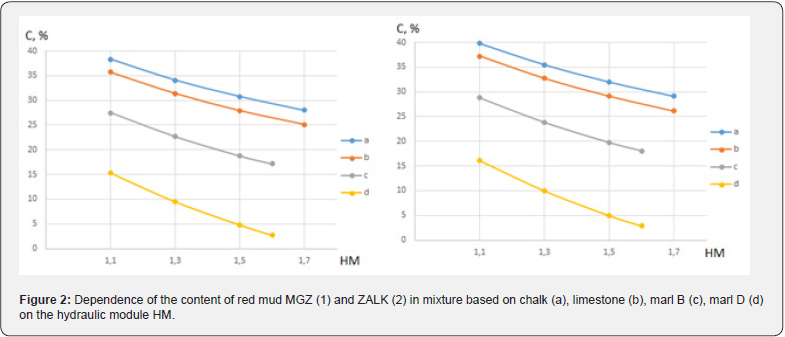
a) In a mixture based on chalk or limestone, the possible content of red mud is quite close: 28.0-38.3 wt. % for the MGZ sample and 29.1-39.8 wt. % for the ZALK sample.
b) In a mixture based on Bakhchisarai marl B, the possible content of red mud is 17.2-27.5 wt. % for the MGZ sample and 18.0-28.8 wt. % for the ZALK sample.
c) In a mixture based on Dubovets marl D, the possible content of red mud is 2.7-15.3 wt. % for the MGZ sample and 2.9- 16.1 wt. % for the ZALK sample.
However, with a sufficiently high possible content of waste in binary mixtures, the binders of them do not meet the recommended for cement level of silica modulus n=1.9-3.0 and alumina modulus p= 0.9-2.0 (Table 2). The practical value can be a binary mixture containing, wt. %: 97.0 Dubovets marl and 3.0 red mud. According to the analysis, such a mixture should provide a binder with the closest to the recommended modulus values.

Chalk - Clay - Red Mud Systems
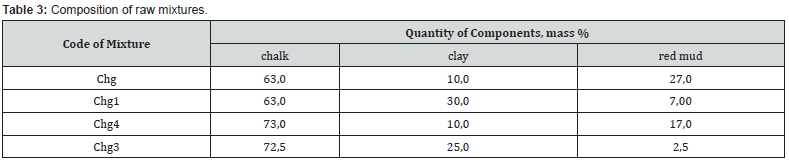
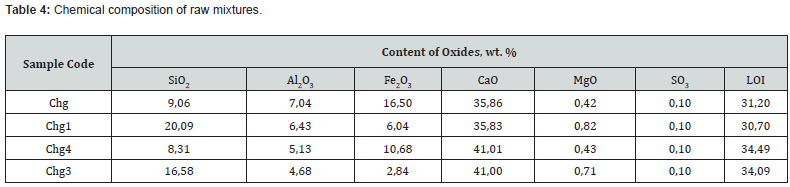
On the basis of computer calculations, it was established that in a three-component mixture based on the system of chalk - Krivinsky clay - red mud, the possible content of the latter is from 2.4 to 18.4 wt. % at HM=1,7 and from 2.4 to 29.1 wt. % at HM=1.1 and increases with a decrease in the hydraulic modulus and the amount of clay (Figure 3). The studied mixtures based on the Krivinsky chalk-clay-red mud system are characterized by differences in the quantitative ratio of components and chemical composition (Tables 3 & 4).
At the hydraulic modulus of НМ=1.1, mixtures of Chg with a maximum of red mud with the same content of CaO from Chg1 clearly differ in the amount of SiO2 (9.1 vs. 20.1 wt.%), the ratio of SiO2: Al2O3 (1.3 vs. 3.1) and Fe2O3 content (16.5 vs. 6.0 wt.%). At the hydraulic modulus НМ=1.7, mixtures of Chg4 with a maximum of red mud at the same content of CaO with Chg3 clearly differ in the amount of SiO2 (8.3 vs. 16.6.1 wt.%), the ratio of SiO2: Al2O3 (1.6 vs. 3 .5) and Fe2O3 content (10.7 vs. 2.8 wt.%). After firing the investigated mixtures, the obtained binders differ in chemical composition (Table 5). The obtained results of testing samples of the studied materials after firing at a maximum temperature of 1100°C indicate certain differences in their binding properties.
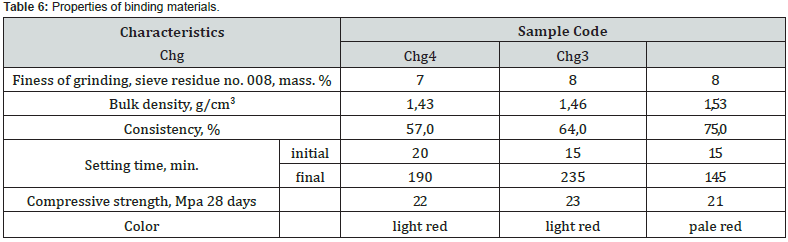
According to the classification of DSTU B V.27-91-99 [28], according to the speed of hardening, the samples of the binder based on the chalk-clay-red mud system belong to the group of fast-hardening (starting time from 15 to 45 min.), characteristic representatives of which are considered to be anhydrite and alumina cement and slag-alkaline binders (Table 6). However, it is obvious that an increase in the content of red mud in the initial mixture leads to a certain increase in the end time and in general the hardening process. Thus, with a hydraulic module of НМ=1.7, with an increase in the content of red sludge from 2.5 to 17 wt. % with the same hardening start time, the end of the process is extended from 145 to 235 min.
According to the speed of hardening of a sample of a solution with a binder based on the system of chalk – clay-red mud, they belong to the group of fast hardening (starting time from 15 to 45 min.), characteristic representatives of which are considered anhydrite and alumina cement and slag-alkaline binders. At the same time, it is obvious that an increase in the content of red mud in the initial mixture leads to a decrease in the time of the beginning and end of hardening of the solution, that is, to a general intensification of this process. Thus, with a hydraulic module of НМ=1.7, with an increase in the content of red mud from 2.5 to 17 wt. % the beginning of hardening of the material decreases from 30 to 25 min., the end - from 235 to 180 min.

The results of the X-ray phase analysis obtained in this work indicate certain differences in the physical and chemical transformations during firing of the studied mixtures, which correlate with the specified chemical composition and depend on the content of red mud in them and the ratio of components (Figures 4 & 5). Thus, after firing to the maximum temperature of 1100°C, a sample of Chg from a mixture containing 27 wt. % of red mud with its quantitative ratio to clay of 2.7: 1, differs from Chg3 by significantly greater development of iron-containing crystalline phases C2F, CF, C4AF. Sample Chg3 from a mixture containing 2.5 wt. % of red mud with its quantitative ratio to clay of 1:10 is characterized by the development of crystalline phases of quartz, silicates (C2S), aluminosilicates (C2AS) and aluminates (СА, С3A) of calcium.

Chalk - Perlite - Red Mud Systems
On the basis of computer calculations, it was established that in a three-component mixture based on the chalk - perlite - red mud system, the possible content of the latter ranges from 2.8 to 28.2 wt. % and increases with a decrease in the hydraulic modulus and amount of perlite (Figure 6). The studied mixtures are characterized by differences in the quantitative ratio of components and chemical composition (Tables 7 & 8). At the same time, mixtures Ch, Ch1, in comparison with those typical for cement production, are characterized by a significantly lower content of carbonate raw materials.
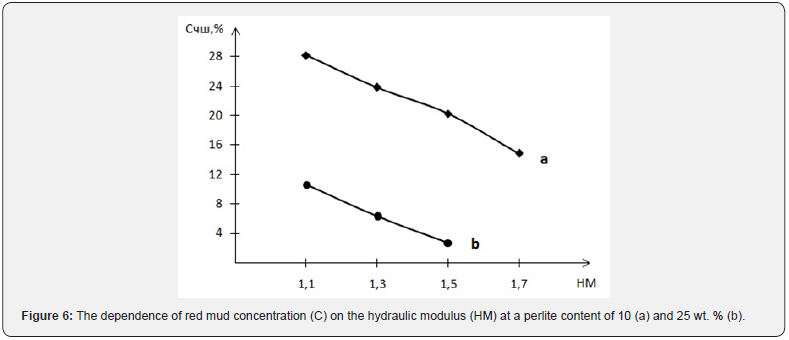
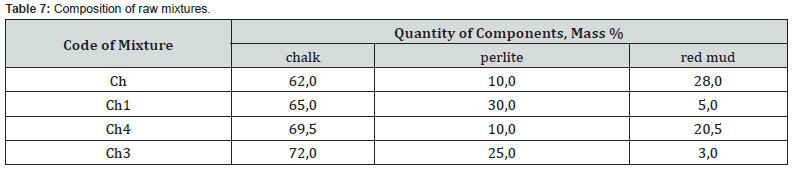
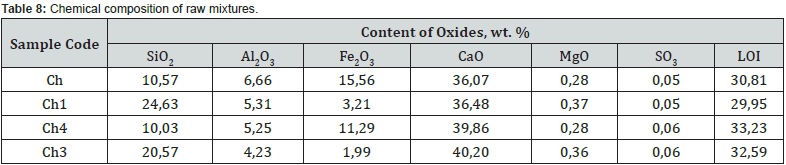
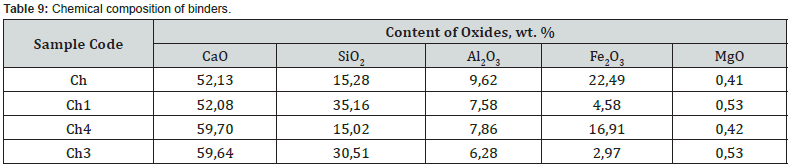
According to the chemical composition, with a hydraulic modulus of НМ=1.1, mixtures Ch and Ch1, with slight differences in the CaO content, differ significantly in the amount of SiO2 (10.6 vs. 24.6 wt.%) and Fe2O3 content (15.6 vs. 3.2 wt. %). Accordingly, the quantitative ratios of oxides SiO2: Al2O3 (1.6 vs. 4.6), which affects the refractoriness of the mixture, and CaO : SiO2 (3.4 vs. 1.5), CaO : Al2O3 (5.4 vs. 6.9), CaO:Fe2O3 (2.3 versus 11.4) differ, which determine the probability of formation of new compounds during firing. At НМ=1.5, the mixtures Ch4, Ch3 differs from Ch, Ch1 in a slightly higher content of CaO, a slightly lower content of silicon, aluminum and iron oxides. At the same time, mixtures Ch4 and Ch3 differ in quantitative ratios of oxides SiO2:Al2O3 (1.9 vs. 4.9), CaO : SiO2 (4.0 vs. 2.0), CaO:Al2O3 (6.5 vs. 9.5), CaO :Fe2O3 (3.5 vs. 20.2). After firing the studied mixtures, the obtained binders differ in chemical composition and silica modulus (Table 9). With the value of the silica modulus in the range of 0.48-3.31 and the alumina modulus in the range of 0.43-2.13, samples Ch1 and Ch3 are noted to be the largest.
According to the classification of DSTU B V.27-91-99, according to the speed of hardening, samples of binders based on the chalkperlite- red system belong to the group of normal hardening (starting time from 45 min. to 2 h), typical representatives of which are Portland cement and slag portland cement (Table 10). However, it is obvious that an increase in the content of red mud in the initial mixture leads to a decrease in the time of the start and end of hardening, that is, to a general intensification of this process. Thus, with the same hydraulic module НМ=1.1, when the content of red increases from 5 to 28 wt. % the beginning of hardening of the material decreases from 70 to 45 min., the end - from 200 to 105 min.
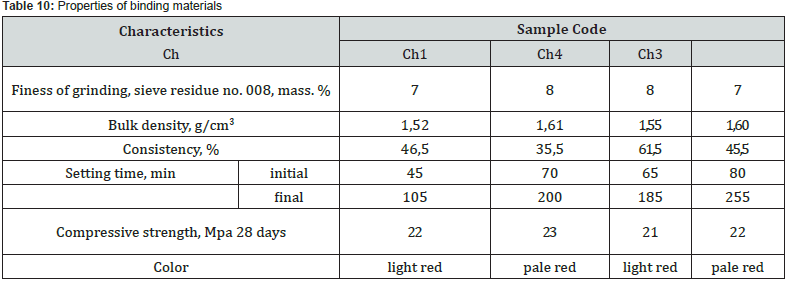
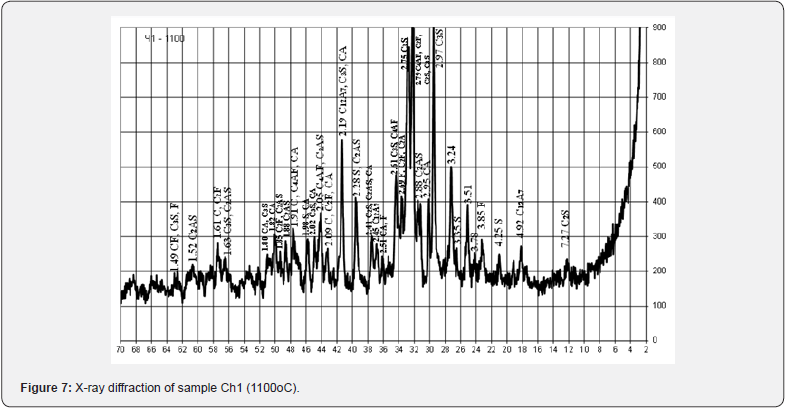
Samples of a solution with a binder based on the chalk-perlitered mud system, according to the speed of hardening, belong to the group of fast-hardening (starting time from 15 to 45 minutes), characteristic representatives of which are considered anhydrite and alumina cement and slag-alkaline binders. samples of the solution in comparison with the samples of the binder are marked by the intensification of the hardening process with a decrease in the time of beginning and end. The results of the X-ray phase analysis obtained in this work indicate certain differences in the physical and chemical transformations during firing of the investigated mixtures, which depend on the content of red mud in them and the ratio of components (Figures 7 & 8).
So, the raw material mixture Ch1 containing 5 wt. % of red mud with a quantitative ratio of 1:6 to perlite is characterized by the presence of a glass phase associated with pearlite, the main rock-forming mineral - calcite, quartz and iron oxides. Raw material mixture Ch, containing 28 wt. % of red mud with its quantitative ratio to perlite of 2.8: 1, is characterized by the presence of a glass phase associated with perlite, the main rockforming mineral - calcite, quartz and iron oxides. After firing at the maximum temperature of 1100°C, the crystal lattices of the main rock-forming minerals of the original raw material mixtures are destroyed and new crystalline phases are formed. At the same time, sample Ch with a relative maximum of red mud is characterized by significantly greater development of iron-containing crystalline phases CF, C2F, C4AF.
Chalk - Pyloquartz - Red Mud Systems
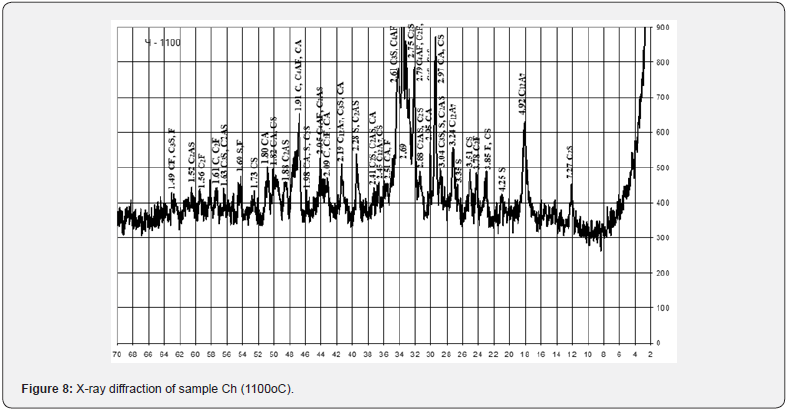
On the basis of computer calculations, it was established that in a three-component mixture based on the system of chalk - pyloquartz - red mud, the possible content of the latter is from 3.5 to 27.7 wt. % and increases with a decrease in the hydraulic modulus and amount of pyloquartz (Figure 9).
The studied mixtures based on the chalk-pyloquartz system are characterized by differences in the quantitative ratio of components and chemical composition (Tables 11 &12). At the same time, mixtures Ch10 and Ch11, in comparison with those typical for cement production, are characterized by a significantly lower content of carbonate raw materials. According to the chemical composition, with the same hydraulic modulus НМ = 1.1, mixtures Ch10 and Ch11, with slight differences in the CaO content, differ significantly in the amount of SiO2 (12.6 vs. 21.6 wt.%) and Fe2O3 content (15.1 vs. 8.6 wt.%). Accordingly, the quantitative ratio of oxides SiO2: Al2O3 (2.4 vs. 7.2), which affects the refractoriness of the mixture, and CaO : SiO2 (2.9 vs. 1.7), CaO:Al2O3 (7.0 vs. 12.2) differ , CaO:Fe2O3 (2.4 versus 4.3), which determine the probability of formation of new compounds during firing.
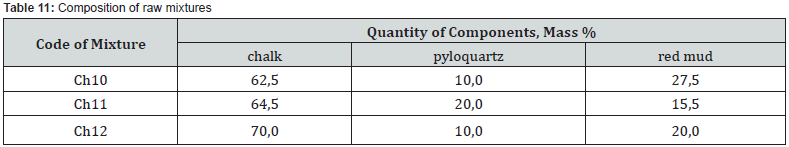
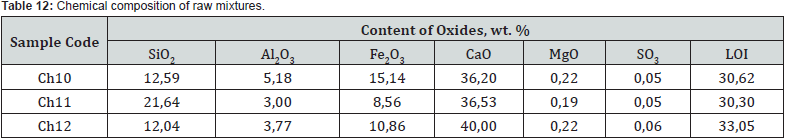
Mixture Ch12 at НМ=1.5 differs from Ch10, Ch11 by a slightly higher content of CaO and is characterized by quantitative ratios of oxides SiO2:Al2O3=3.2, CaO : SiO2 = 3.3, CaO : Al2O3 = 10.6, CaO:Fe2O3 = 3.7. After firing the investigated mixtures, the obtained binders differ in chemical composition and silica modulus (Table 13). With a silica modulus value in the range of 0.62-1.87, sample Ch11 is the largest, and the alumina modulus of the samples is at the level of 0.34-0.35. According to the classification of DSTU B V.27-91-99, after firing at the maximum temperature of 1100oC, the studied samples of the binder based on the chalk - pyloquartz system belong to different groups according to the hardening speed (Table 14). With a relatively lower content of red mud and the largest content of pyloquartz with their quantitative ratio of 1.5: 2, sample Ch11 belongs to the ultra-fast hardening group (starting time no later than 15 minutes), which is considered typical for expanding and tensioning cements.

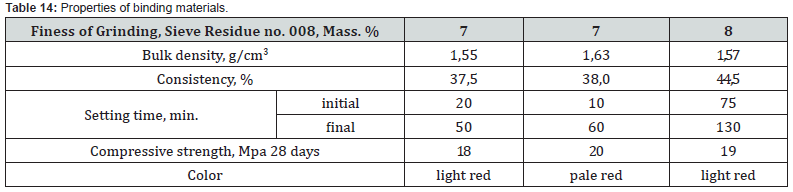
With the relatively highest content of red mud and its quantitative ratio with pyloquartz 2.8:1, sample Ch10 belongs to the group of fast hardening (starting time from 15 to 45 min.), which is considered typical for anhydrite and alumina cements. With a quantitative ratio of red mud to pyloquartz of 2:1, sample Ch12 belongs to the normal-hardening group (onset period from 45 min. to 2 h), characteristic representatives of which are Portland cement and Portland slag cement. The obtained results of the X-ray phase analysis indicate certain differences in the physical and chemical transformations during firing of the studied mixtures, which depend on the content of red sludge in them and the ratio of components (Figures 10-12).
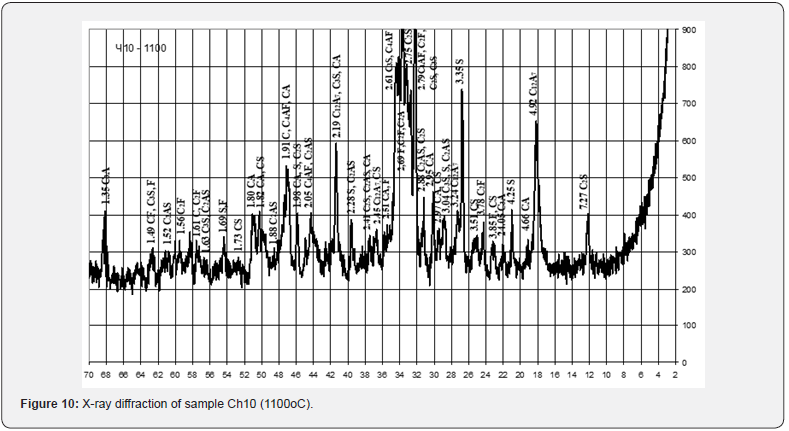
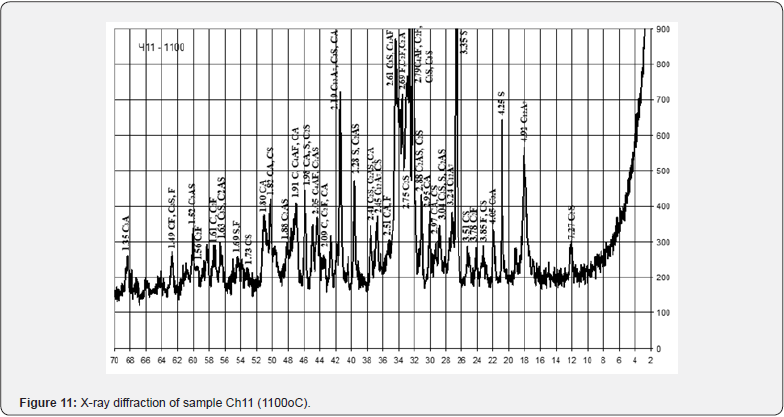
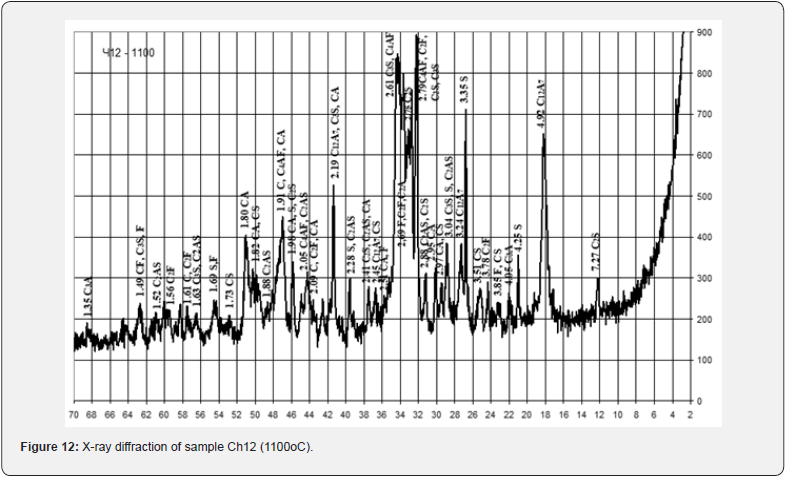
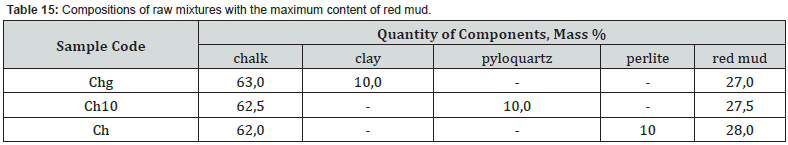
Thus, after firing to the maximum temperature of 1100°C, sample Ch10 from a mixture containing 27.5 wt. % of red mud with its quantitative ratio to pyloquartz 2.8 : 1, differs from Ch11 from a mixture containing 15.5 wt. % of red slime with its quantitative ratio to pyloquartz 1.5 : 2, significantly greater development of iron-containing crystalline phases C4AF, C2F. Sample Ch11 is characterized by greater development of quartz, aluminosilicates (C2AS), aluminates (CA) and calcium silicates (C2S). The possibility of introducing 27.0 - 28.0 wt. % to the composition of raw mixtures based on chalk-polymineral clay, chalk-perlite, chalk-quartz systems for the production of mineral binder when fired at a maximum temperature of 1100°C (Table 15).
Discussion
These studies correspond to the principle of modern materials science about the relationship “composition-structureproperties”. With the use of computer calculations, it was possible to determine the quantitative limits of utilization of one of the multi-use industrial wastes - red sludge for the production of an energy-saving type of low-temperature fired cement. At the same time, the features of the use of red mud are considered when varying the composition of mixtures with natural raw materials of different genesis.
It is obvious that the obtained results do not solve the entire set of problems for the utilization of red mud as a man-made raw material in the production of cement, but it is a significant positive step in this direction. Probably, certain weaknesses of the submitted work include the limitation of red mud samples with waste from Ukrainian enterprises, which is related to the practical capabilities of the authors in modern conditions.
Conclusion
a) The use of red mud as a component of the raw material mixture for the production of mineral binding materials is one of the directions for the utilization of multi-tonnage non-ferrous metallurgy waste.
b) In the production of low-temperature fired cement (≤1200°С) with a hydraulic modulus of НМ=1.1-1.7, the possible content of waste in a three-component mixture is:
i. 2.4-29.1 wt. % in the “chalk - clay - red mud” system with provision, at the indicated minimum, the values of siliceous n=2.21-2.35 and alumina p=1.65-1.84 modules
ii. 4.9-28.2 wt. % in the “chalk – perlite – red mud” system with provision, at 4.9-5.7 wt. %, values of siliceous n=2.23-2.89 and alumina p=1.16-1.66 modules
iii. 3.5-27.7wt. % in the “chalk - pyloquartz - red mud” system with provision at 4.9- 15.6 wt. % values of silica n=1.90- 2.89 and alumina p=0.35-1.66 modules.
c) The manufactured materials belong to the group of fast-hardening materials but differ in the intensity of the process: hardeners based on the “chalk-clay-red mud” system are characterized by a longer time to the end of hardening, and those based on the “chalk-perlite-red mud” system - by a longer time to the beginning of hardening. They belong to the groups of mineral binders with reduced strength (10-30 MPa) but will meet or exceed the compressive strength indicators regulated for natural and Roman cement.
d) The marked color of the obtained cement, which increases with an increase in the content of red mud, improves the decorative properties of the material in accordance with the requirements of architecture.
e) The possibility of reduction to 62-65 wt. % of the content of the carbonate component in the original raw material mixtures is important for solving the issues of resource saving and ecology.
References
- Pashchenko ОО, Serbin VP, Starchevska ОО (1995) Binding materials. Кyiv: Vyshch shk pp. 440.
- Ghosh SN (2003) Advances in Cement Technology: Chemistry, Manufacture and Testing. Taylor & Francis pp. 828.
- Winter NB (2012) Understanding Cement WHD Microanalysis Consultants Ltd. pp. 206.
- Harris J, Roach B (2021) Environmental and Natural Resource Economics. A Contemporary Approach (5th Edn), Routledge, UK pp. 716.
- Jhariya MK, Meena RS, Banerjee A, Meena SN (2021) Natural Resources Conservation and Advances for Sustainability. (1st) Elsevier pp. 650.
- Sviderskyy VА, Chernyak LP, Salnik VG, Pakhomova VМ, Sikorskyy ОО (2015) Resource conservation and raw materials of silicate production. Education manual - Кyiv: Politekhnika. p. 92.
- Udachkin IB, Pashchenko AA, Chernyak LP, Zakharchenko PV, Semididko AS, et al. (1988) Comprehensive development of the raw material base of the building materials industry. Budivelnyk, Kyiv.
- Allen DT, Benmanesh N (1994) Wastes as Raw Materials. The Greening of Industrial Ecosystems. Washington: National Academy Press pp. 69-89.
- Dvorkin LY, Dvorkin OL (2007) Building materials from industrial waste: a study guide, Feniks, Rostov on D.
- Mossur PM, Negoda SV (2007) Technogenic mineral raw materials and its use in Ukraine. GIИАB 6: 299-307.
- Ramesh M, Karthic KS, Karthikeyan T, Kumaravel A (2014) Construction materials from industrial wastes-a review of current practices. International Journal of Environmental Research and Development 4(4): 317-324.
- DSTU B V 2.7-46 (2011) Cements for general construction purposes. Specifications Minregionbud of Ukraine, Kyiv.
- UNE EN 197-1 (2011) Cement - Part 1: Composition, specifications and conformity criteria for common cements
- Pashchenko AA, Myasnikova EA, Evsutin ER (1990) Energy-saving and waste-free technologies for the binders production. Vyshch shk Kyiv.
- Dvorkin LY (2022) Effective ash-containing cements, concretes and mortars: monograph. Каrаvеlа, Kyiv.
- Chalov VI, Kravchino ОP, Еkimov SV (1984) Use of non-ferrous metallurgy waste for the production of building materials – М VNIIESM, p. 29.
- Stephen KR (2014) Making the Most of Red Mud. - Chemical & Engineering News 92(8): 33-35.
- Gubina VG, Kadoshnikov VM (2005) The red mud of the Mykolaiv Alumina Plant is a valuable technogenic raw material. Geologo-mineralogichnyy visnyk 2: 122-126.
- Harekrushna S, Subash CM, Santosh KS, Ananta PC, Himanshu SM (2014) Progress of Red Mud Utilization: An Overview. American Chemical Science Journal 4(3): 255-279.
- Wang P, Liu DY (2012) Physical and chemical properties of sintering red mud and Bayer red mud and the implications for beneficial utilization. Materials 5: 1800-1810.
- Say VI, Chernyak LP (1985) Improving the technology of building ceramics. Znanie, Kyiv.
- Chernyak LP, Коmskyy GZ, Тrubachev VI (1986) Ceramic finishing materials based on iron-containing industrial waste. Production and use of effective finishing materials in construction - L: Znanie pp. 44-48.
- Melnyk L, Svidersky V, Chernyak L, Dorogan N (2018) Aspects of making a composite material when using red mud. Eastern-European Journal of Enterprise Technologies 6(92): 23-28.
- Liu XM, Zhang N (2011) Utilization of red mud in cement production: A review. Waste Management & Research 29: 1053-1063.
- Ribeiro DV, João AL, Marcio RM (2011) Potential use of natural red mud as pozzolan for Portland cement. Mat Res 14(1).
- Senff L, Modolo RCE, Silva AS, Ferreira VM, Hotza D, et al. (2014) Influence of red mud addition on rheological behavior and hardened properties of mortars. Construction and Building Materials 65: 84-91.
- Sviderskyy VА, Chernyak LP, Sanginova ОV, Dorogan NО, Tsybenko МU (2017) Low-temperature binder technology software. Building materials and products 1-2(93): 22-24.
- DSTU B V.2.7-91-99 (1999) Mineral binders. Classification, Derzhbud of Ukraine, Kyiv.






























