Interaction of Heteroatom-Containing Organic Compounds with Bulk Nickel Alloy in a Closed Reactor System
Roman M Kenzhin1, Yurii I Bauman1, Alexander M Volodin1, Ilya V Mishakov1,2, Aleksey A Vedyagin1,2,*
1Boreskov Institute of Catalysis SB RAS, Novosibirsk, Russian Federation
2 National Research Tomsk Polytechnic University, Tomsk, Russian Federation
Submission: March 14, 2018; Published: March 23, 2018
*Corresponding author: Aleksey A Vedyagin, Boreskov Institute of Catalysis SB RAS, Novosibirsk, Russian Federation, Email: vedyagin@catalysis.ru
How to cite this article: Kenzhin R M, Bauman Y I, Volodin A M, Mishakov I V, Vedyagin A A. Interaction of Heteroatom-Containing Organic Compounds with Bulk Nickel Alloy in a Closed Reactor System. JOJ Material Sci. 2018; 4(2): 555633. DOI: 10.19080/JOJMS.2018.04.555633
Abstract
Catalytic chemical vapor deposition (CCVD) of different organic compounds (butyl iodide, chlorobenzene and melamine) over bulk nickel-containing items in a closed reactor system was overviewed. Scanning electron microscopy (SEM) and ferromagnetic resonance (FMR) spectroscopy were applied to characterize the samples. The process of self-disintegration of bulk metal precursor was shown to require a presence of both hydrogen and heteroatom sources in a reaction medium. Chlorobenzene and butyl iodide contain both hydrogen and halogen in an appropriate ratio. Melamine was considered as a nitrogen-containing organic compound, which is proper to synthesize the nitrogen-doped carbon nanofibers. Disintegration of nichrome wire with formation of dispersed particles catalyzing the growth of carbon nanofibers in the presence of butyl iodide was found to take place at 475°C, while in the cases of chlorobenzene and melamine the temperature required to initialize the process was 550 and 560°C, correspondingly.
Keywords: CCVD; halogenated organics; melamine; closed reactor system; RAPET; bulk nickel alloy
Abbreviations: CCVD: Catalytic Chemical Vapor Deposition; SEM: Scanning Electron Microscopy; FMR: Ferromagnetic Resonance; CCVD: Catalytic Chemical Vapor Deposition; RAPET: Reactions under Autogenic Pressure At Elevated Temperature
Introduction
The nanostructured forms of carbon (carbon nanotubes and nanofibers) are known to be applied in wide areas of modern science and technology [1-4]. There are a lot of methods to produce them, among which the catalytic chemical vapor deposition (CCVD) should be especially mentioned [5-7]. Usually, the dispersed particles of metals of iron subgroup (Fe, Ni, Co) are used for controllable synthesis of such materials via CCVD process. Hydrocarbons of different nature can be used as a carbon source [8]. It allows one to consider the nanostructured carbon synthesis via decomposition of the heteroatom-substituted hydrocarbons, thus changing significantly the properties of the carbon product. In chemical industry, the halogenated organic compounds, being a high demanded intermediate for polymers and plastics production, are manufactured on a large scale. Unfortunately, the processes conventionally used to obtain these chemicals have an important from ecological point of view drawback - they are accompanied with formation of toxic wastes containing of a mixture of organohalogens. Hereby, processing of these compounds into carbon nanostructures is of a great practical interest [9-11]. Recently we have reported that bulk nickel and its alloys can serve as a self-organizing catalyst decomposing as individual halogenated compounds as their complex mixtures [12-15]. On the other hand, nitrogen-doped carbon nanofibers possessing a variety of unique properties also attract a growing attention. In order to synthesize them via CCVD process, a nitrogen-containing compound should be added to the reaction feed of hydrocarbons to be decomposed. The use of a single compound containing both the carbon and nitrogen sources allows one to simplify the manufacturing procedure. In the present paper, the synthesis of the nanostructured carbon products via CCVD of different heteroatom-containing compounds (butyl iodide, chlorobenzene and melamine) over bulk nickel-containing items in a closed reactor system, when no gaseous products are removed from the reaction volume, will be reviewed. As it was reported by Prof. A. Gedanken, this approach, also known as Reactions under Autogenic Pressure at Elevated Temperature (RAPET), looks very promising to prepare the structured materials of a different nature [16-18].
A Concept of a Closed Reactor System
The experiments in a closed reactor system are carried out in the quartz ampoules (d = 4-5 mm, V = 0.2 ml) serving as a reactor. As it shown in (Figure 1), a piece of nichrome wire (d = 0.1 mm) of about 0.2-0.3 mg is placed in an ampoule together with 2-3 mg of carbon precursor (butyl iodide, chlorobenzene or melamine). The ampoule with reaction mixture is soldered and then brought to thermal treatment at certain temperature. The accuracy of temperature measurements is ±2°C. The FMR spectra of samples in closed ampoules are registered at a room temperature using an experimental setup based on an ERS-221 ESR spectrometer described in [19]. The processing of the spectra obtained is performed using ESR_CAD software developed earlier. Then, the ampoules are carefully opened for further investigation by means of a scanning electron microscopy (SEM) combined with EDX analysis (JSM 6460, Jeol, Japan). As it was reported recently [20], realization of CCVD process in a close system was shown to suppress totally the formation of nanostructured carbon during the interaction of metallic nickel with the halogen-substituted hydrocarbons (C6F6, C6Cl6). Instead of carbon product, microcrystals of nickel halogenides were observed. The key factor for disintegration of bulk metal items with formation of nanostructured carbon is a simultaneous presence of halogen and hydrogen sources. From this point of view, both chlorobenzene and butyl iodide can be considered as appropriate precursors [21,22]. Decomposition of melamine attracts an interest since its molecule contains heteroatom of different nature [23].
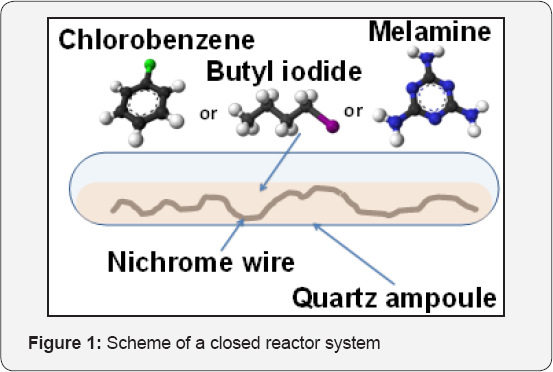
Starting Point of the Interaction
Ferromagnetic resonance spectrometry was used as an efficient method to study the very beginning stages of the disintegration process in a closed reactor system, when disperse nickel particles are expected to appear. The main advantage of this approach is that the nichrome wire does not exhibit ferromagnetism at room temperature, and thus does not give a FMR signal (Figure 2). In the case of chlorobenzene decomposition, the process of self-disintegration of the bulk nichrome begins at 550°C. A wide signal with gav=2.3, which is typical for FMR spectra of dispersed nickel particles, along with intensive narrow singlet (g=2.003) characteristic for various carbon materials was found to appear at 560°C. Heating the ampule up to 610°C increases significantly the intensity of FMR signal, indicating that larger amount of the disperse metal particles was resulted from the disintegration process. At higher temperatures, when more carbon is formed, the registration of FMR spectra was complicated due to a strong microwave absorption by the sample. When butyl iodide was used as a precursor compound, the corresponding FMR signal has appeared after the ampule with reactants was heated up to 475 °C. While this temperature is lower by 75°C than in the case of chlorobenzene, it significantly exceeds the temperature for decomposition of hexamethylbenzene and hexafluorobenzene mixture (340°C) [20]. The noticeable FMR signal was observed after the heating at 580°C (Figure 2). An interaction of melamine with nichrome wire inside the sealed ampule (closed reactor system) along with self-organization of ferromagnetic dispersed Ni particles was found to take place at 560°C, when the weak FMR signal characterized by wide single band with typical for Ni parameters g ~ 2.3 and AH = 500-1100 G has appeared.
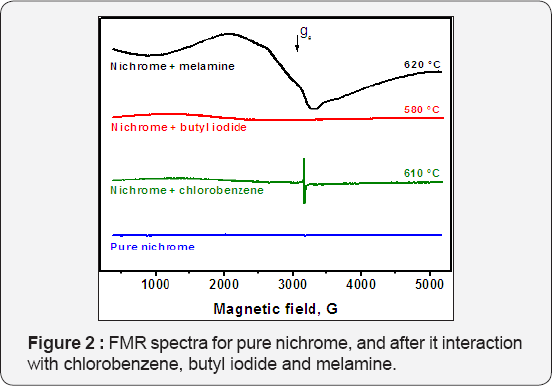
Microscopic Characterization of the Products
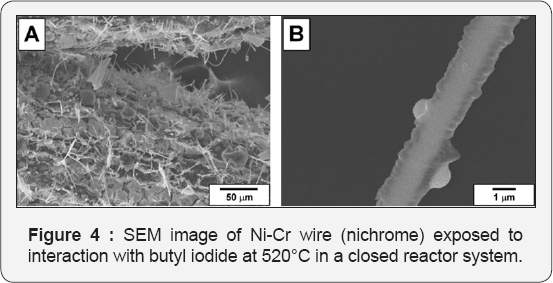
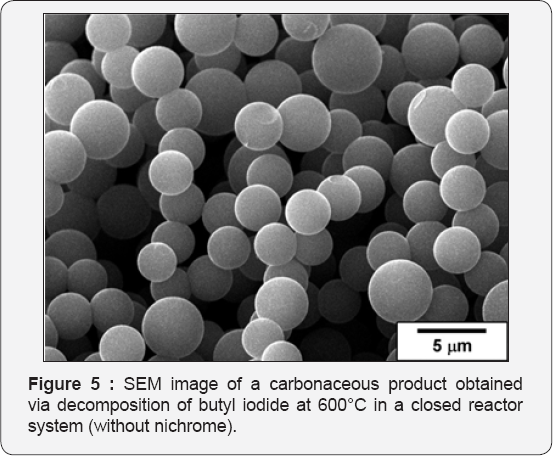
SEM images for the nichrome samples after interaction with chlorobenzene at 730°C are shown in (Figure 3). Thick carbon fibers of about 3-5 microns in diameter covering the surface of the alloy are well seen. According to data of EDX analysis, these fibers contain trace amount of chlorine. It should be emphasize that no self-disintegration ofthe bulk nichrome alloy or formation of the carbon nanofibers was observed in a closed reactor system when benzene or hexacholobenzene was used as an organic substrate instead of chlorobenzene (Figure 4A). demonstrates the corresponding SEM images of the NiCr sample after its interaction with butyl iodide at 520°C. The resulting material is represented by a mixture of various structures. The most interesting among them are faceted rods of 50 microns in length and 2 microns in diameter (Figure 4B). The composition of these structures, in accordance with EDX analysis, corresponds to NiI2 stoichiometry. The observed microcrystals can exist in a limited range of temperatures and undergo complete destruction after calcination at temperature of 680°C and above. It is worth to note that the transformation of organic compounds in a closed reactor system without Ni-Cr sample is accompanied by formation of the uniform spherical carbon particles with characteristic size of ~ 3 microns. An example SEM image of the material obtained after butyl iodide decomposition at 600°C inside the empty sealed ampoule is shown in (Figure 5).

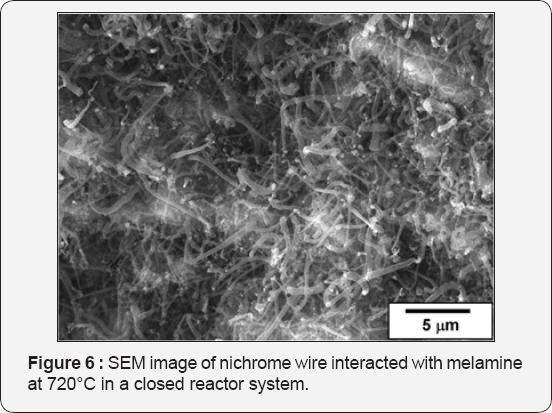
Additionally, decomposition of melamine as a nitrogen- containing organic compound was performed in order to obtain the N-doped carbon nanofibers. As it was already mentioned, a noticeable interaction of melamine with nichrome starts at 560°C. After 2 h of such interaction, the surface of the wire became covered with nodes, which are considered to play a role of nucleus of the growing nanofibers. These nodes predominantly consist of carbon (about 38-40 at.%) and nitrogen (~58-60 at.%) with small impurities of oxygen (less than 2 at.%). An increase of reaction temperature intensifies the CCVD process, resulting in a larger yield of the nanostructured carbon. SEM image for the sample exposed to interaction with melamine at 720°C is presented in (Figure 6). As seen, the carbon nanofibers of few tens nm cover the wire surface. The content of nitrogen in these fibers, which are quite longer if compare with at 560°C, is about 5-10 at.%. The main feature of melamine decomposition comparing with CCVD of chlorobenzene or butyl iodide is that the quartz ampule remains clear and transparent after the experiments. Piece of wire covered with N-CNF is well seen on its bottom. No side solid products were formed, oppositely to decomposition of halogen-containing substrates when ampule walls were covered with non-catalytic carbon. Thereby, it can be concluded that all melamine has been selectively and completely reacted with nichrome wire.
Conclusion
In the present paper, catalytic chemical vapor deposition of chlorobenzene, butyl iodide and melamine over bulk nichrome alloy was discussed. The current results testify towards previously made assumption [20] that the process of selfdisintegration of bulk metal precursor requires presence of both hydrogen and heteroatom sources in a reaction medium. Herewith, hydrogen and halogen might be contained in one compound (like in chlorobenzene and butyl iodide). In the case of chlorobenzene, no intermediate solid-phase halogenides were detected after the experiments in a closed reactor system. Formation of thick carbon fibers of about 3-5 microns in diameter was observed. The starting temperatures of carbon nanofibers formation correlate well with FMR data on disperse Ni particles appearance (initial point of bulk nichrome selfdispersion process). In the case of butyl iodide, among the variety of structured products, the formation of faceted rods of 50 microns in length and 2 microns in diameter was found. The nitrogen-doped CNFs were synthesized using melamine as a substrate containing both carbon and nitrogen. Disintegration of nichrome resulted in formation of the dispersed Ni particles catalyzing growth of CNFs was shown to take place at 560°C and above. Significant yield of the nanostructured carbon was obtained at 720°C. Resulted N-doped carbon nanofibers contain up to 10 at.% of nitrogen.
Acknowledgement
Financial support by the Russian Foundation for Basic Research (project 16-33-60034) is acknowledged with gratitude.
References
- He P, Liu L, Song W, Xiong G, Fisher TS, et al. (2015) Large-scale synthesis and activation of polygonal carbon nanofibers with thin ribbon-like structures for supercapacitor electrodes. RSC Adv 40: 31837-31844.
- Mamun A Al, Ahmed YM, Al Khatib MFR, Jameel AT, Abdul RAS, et al. (2015) Lead sorption by carbon nanofibers grown on powdered activated carbon - kinetics and equilibrium. Nano 10(2): 15500171550025.
- Lin JF, Mohl M, Nelo M, Toth G, Kukovecz A, et al. (2015) Facile synthesis of nanostructured carbon materials over RANEY® nickel catalyst films printed on Al2O3 and SiO2 substrates. J Mat Chem C 3: 1823-1829.
- Sainio S, Jiang H, Caro MA, Koehne J, Lopez Acevedo O, et al. (2016) Structural morphology of carbon nanofibers grown on different substrates. Carbon 98: 343-351.
- Bouchet Fabre B, Pinault M, Foy E, Hugon MC, Minea T, et al. (2014) Interface study between nanostructured tantalum nitride films and carbon nanotubes grown by chemical vapour deposition. Appl Surf Sci 315: 510-515.
- Wang H, Wang Y, Xue R, Kang L, Li X (2012) Fabrication and electron field-emission of carbon nanofibers grown on silicon nanoporous pillar array. Appl Surf Sci 261: 219-222.
- Jana M, Sil A, Ray S (2016) Chemical nature of catalysts of oxide nanoparticles in environment prevailing during growth of carbon nanostructures by CCVD. B Mater Sci 39(7): 1783-1790.
- Bauman YI, Mishakov IV, Vedyagin AA, Ramakrishna S (2017) Synthesis of bimodal carbon structures via metal dusting of Ni-based alloys. Mater Lett 201: 70-73.
- Menini C, Park C, Shin EJ, Tavoularis G, Keane MA (2000) Catalytic hydrodehalogenation as a detoxification methodology. Catal Today 62(4): 355-366.
- Keane MA, Jacobs G, Patterson PM (2006) Ni/SiO2 promoted growth of carbon nanofibers from chlorobenzene: Characterization of the active metal sites. J Colloid Interf Sci 302(2): 576-588.
- Nieto-Marques A, Valverde JL, Keane MA (2007) Catalytic growth of structured carbon from chloro-hydrocarbons. Appl Catal A-Gen 332(2): 237-246.
- Bauman YI, Mishakov IV, Vedyagin AA, Serkova AN, Gromov AA (2017) Kinetic Features of the Carbon Erosion of a Bulk NiCr Alloy during the Catalytic Decomposition of 1,2-Dichloroethane. Kinet Catal 58(4): 448-454.
- Vedyagin AA, Mishakov IV, Bauman YI (2017) Bulk Metal Alloys as a Precursor of Dispersed Particles Catalyzing Growth of Carbon Nanofibers. JOJ Material Sci 2: 555594.
- Bauman YI, Lysakova AS, Rudnev AV, Mishakov IV, Shubin YV et al. (2014) Synthesis of Nanostructured Carbon Fibers from Chlorohydrocarbons over Bulk Ni-Cr Alloys. Nanotechnol Russia 9(7- 8): 380-385.
- Bauman YI, Mishakov IV, Vedyagin AA, Dmitriev SV, Mel'gunov MS, et al. (2012) Processing of Organochlorine Waste Components on Bulk Metal Catalysts. Catal Ind 4(4): 261-266.
- Pol SV, Pol VG, Gedanken A (2004) Reactions under autogenic pressure at elevated temperature (RAPET) of various alkoxides: formation of metals/metal oxides-carbon core-shell structures. Chem Eur J 10(18): 4467-4473.
- Pol SV, Pol VG, Gedanken A (2005) Novel synthesis of high surface area silicon carbide by RAPET (reactions under autogenic pressure at elevated temperature) of organosilanes. Chem Mater 17(7): 17971802.
- Butovsky E, Perelshtein I, Nissan I, Gedanken A (2013) Fabrication, characterization, and printing of conductive ink based on multi coreshell nanoparticles synthesized by RAPET. Adv Funct Mater 23: 57945799.
- Heroux DS, Volodin AM, Zaikovskii VI, Chesnokov VV, Bedilo AF, et al. (2004) ESR and HRTEM study of carbon-coated nanocrystalline MgO. J Phys Chem B 108(10): 3140-3144.
- Kenzhin RM, Bauman YI, Volodin AM, Mishakov IV, Vedyagin AA (2016) Structural self-organization of solid-state products during interaction of halogenated compounds with bulk Ni-Cr alloy. Mater Lett 179: 3033.
- Kenzhin RM, Bauman YI, Volodin AM, Mishakov IV, Vedyagin AA (2017) Interaction of Bulk Nickel and Nichrome with Halogenated Butanes. Reacn Kinet Mech Catal 122(2): 1203-1212.
- Kenzhin RM, Bauman YI, Volodin AM, Mishakov IV, Vedyagin AA (2018) Synthesis of carbon nanofibers by catalytic CVD of chlorobenzene over bulk nickel alloy. Appl Surf Sci 427: 505-510.
- Kenzhin RM, Bauman YI, Volodin AM, Mishakov IV, Vedyagin AA (2017) One-step synthesis of nitrogen-doped carbon nanofibers from melamine over nickel alloy in a closed system. Chem Phys Lett 685: 259-262.






























