Adsorptive Removal of Phosphorus using Metal-Loaded Biosorbents from Aquatic Environment
Katsutoshi Inoue1*, Hiroyuki Harada2, Kedar Nath Ghimire3, Biplob Kumar Biswas4, Hidetaka Kawakita1, Keisuke Ohto1
1Department of Applied Chemistry, Faculty of Science & Engineering, Saga University, Japan
2Department of Environmental Science, Prefectural University of Hiroshima, Japan
3Central Department of Chemistry, Tribhuvan University, Nepal
4Department of Chemical Engineering, Jessore University of Science and Technology, Bangladesh
Submission: February 15, 2018; Published: February 23, 2018
*Corresponding author: Katsutoshi Inoue, Department of Applied Chemistry, Faculty of Science & Engineering, Saga University, Honjo-machi, Saga, Japan; Tel: +81 9052907575; Email: kanokol921@gmail.com
How to cite this article: Katsutoshi Inoue, Hiroyuki Harada, Kedar Nath Ghimire, Biplob Kumar Biswas, Hidetaka Kawakita, et al. Adsorptive Removal of Phosphorus using Metal-Loaded Biosorbents from Aquatic Environment. JOJ Material Sci. 2018; 4(2): 555632. DOI: 10.19080/JOJMS.2018.04.555632
Abstract
Adsorption gel for metal ions was prepared from orange juice residue in a simple manner by interacting with calcium hydroxide. Heavy metal ions such as lead(II), iron(III) and copper(II) were selectively adsorbed over other metal ions such as zinc(II), cadmium(II) and manganese(II) at low pH. Loading this gel with high valent metal ions such as lanthanum(III), cerium(III) and zirconium(IV) resulted in effective and selective adsorption of phosphate from water containing excess concentrations of other anionic species such as sulfate, carbonate and chloride. Zirconium(IV) was the most effective among the metal ions. Among the phosphorus compounds, phosphate was more strongly adsorbed on zirconium(IV)-loaded gel than phosphite and hypophosphite. The adsorption capacity for phosphate on the zirconium(IV)-loaded gel was the highest among adsorbents that have been reported in literature, including other zirconium(IV)-containing adsorbents. The effectiveness of this gel was verified by applying it to actual wastewater samples containing trace concentrations of phosphorus.
Keywords: Removal of phosphorus; Adsorption; Biosorbents; Orange juice residue; Loading of high-valent metal ions
Abbreviations : OJR :Orange Juice Sesidue; SOJR: Saponified Orange Juice Sesidue
Introduction
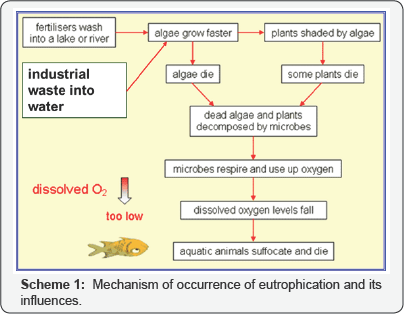
Phosphorus is in a group 15, the nitrogen family, together with nitrogen, arsenic, antimony, and bismuth. Although the main valencies of phosphorus are three and five, trivalent phosphorus is chemically unstable and prone to oxidation into the penta- valent state. In nature, these elements exist as oxo-anions such as phosphate. Phosphorus and nitrogen discharged into the aquatic environment disturbs the balance of organisms present in water and affect water quality, mainly through depletion of the dissolved oxygen levels caused by the decay of algae. Depletion of the oxygen level in confined water bodies such as bays, lakes, and ponds causes eutrophication as depicted in Scheme 1 [1], which has a harmful effect on fish and other aquatic life, resulting in reduced biodiversity and unfavorable environmental conditions for human health. The permissible maximum concentration of phosphorus in effluents in Japan is presently 16 mg dm-3 [2]. However, phosphorus is an essential elements for all organisms and is used in fertilizers, various chemicals and so forth in large amount. The availability of the main resource of phosphorus, phosphate rock, is decreasing drastically at present, and recovery and reuse of phosphorus from the environment is becoming very important.
To meet the environmental standards for phosphorus detailed above, numerous treatment methods such as co-precipitation, ion exchange, ultrafiltration, and adsorption have been proposed, and some of these have been conducted on commercial scales. The most extensively commercialized technique in Japan is crystallization using calcium or magnesium, which are termed as HAP (hydroxyapatite) and MAP (magnesium ammonium phosphate) methods, respectively [3].
In the HAP method, phosphate in aqueous solutions is recovered as crystalline of hydroxyapatite (Ca10(OH)2(PO4)6) according to the following reaction.
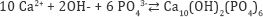
The MAP method is used for solutions containing both phosphorus and ammonia, which are recovered as crystalline struvite (magnesium ammonium phosphate, MgNH4PO4.6H2O) according to the following reaction.
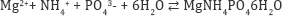
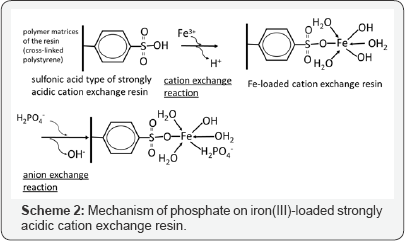
Although these crystallization methods are suitable for wastewater containing high concentrations of phosphorus, they are unsuitable for wastewaters containing low or trace concentrations of phosphorus. Adsorption and ion exchange technologies are much more suitable than other methods for removing low or trace concentrations of phosphate from aqueous solutions. However, commercially available adsorbents such as anion exchange resins and activated carbons are not selective for phosphate over coexisting anionic species in aqueous solutions. For the adsorptive removal of anionic species of arsenic and phosphorus, cation exchange materials such as strongly acidic cation exchange resins loaded with high-valent metal cations (e.g. ferric, Fe3+) with high affinity for the anionic species in question, have been proposed [4]. As an example, the mechanism of adsorption of dihydrogen phosphate (H2PO4-) on a sulfonic acid strongly acidic cation exchange resin loaded with Fe3+ is shown in Scheme 2. In this case, among three positive charges of Fe3+, only one is neutralized by a sulfonic acid functional group. It is difficult for other sulfonic groups to approach Fe3+ because of strong steric hindrance from the cross linked matrix (i.e., cross linked polystyrene in this case). The other two positive charges of Fe3+ are neutralized by anionic species in the aqueous solution, such as hydroxyl ions. These anionic species are then substituted by the target anionic species (e.g., phosphate). Consequently, these anionic species can be easily adsorbed and desorbed by changing the pH. For the purpose of developing environmentally benign technologies for removing and recovering these anionic species, we studied the adsorptive recovery and removal of these species using natural biomass wastes instead of synthetic materials such as ion exchange resins produced from petroleum. There are a variety kinds of natural organic polymeric materials containing functional groups that can adsorb cationic metal ions, including acidic polysaccharides such as alginic acid and pectic acid [5], basic polysaccharide such as chitosan [6] and various proteins [7]. Development of adsorbents using various biomass wastes has attracted much attention in recent years [8].
Adsorption of Metal Ions on Acidic Polysaccharides
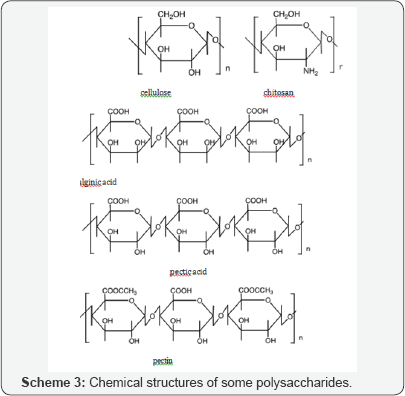
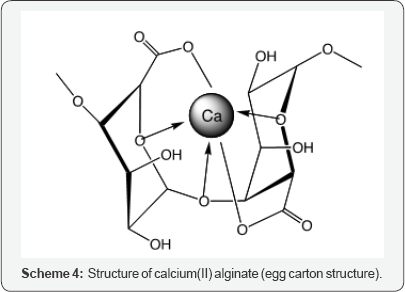
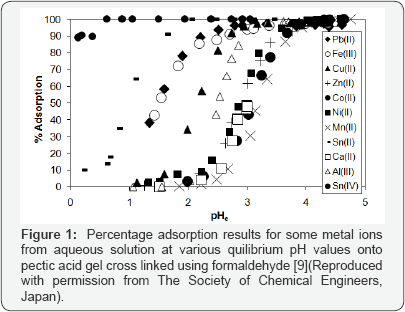
Scheme 3 shows the chemical structures of some polysaccharides, including alginic acid and pectic acid, which are typical acidic polysaccharides containing carboxylic acid functional groups. Alginic acid is contained in sea-weeds as a major component together with cellulose as calcium(II) alginate, which has a well-known egg carton structure as shown in Scheme 4. On the other hand, pectic acid occurs in fruits such as oranges and apples as pectin, which is formed from pectic acid by partial methyl-esterification (Scheme 3). It is easily saponified by alkali such as sodium hydroxide and calcium hydroxide as shown in Scheme 5. Divalent metal ions such as calcium(II) form stable five-membered chelates with alginic acid and pectic acid via coordination with oxygen atoms of carboxylic groups and pyranose rings of the polysaccharides as well as ether oxygens between pyranose rings as shown in Scheme 4. Figure 1 shows the percentage adsorption (% adsorption) results for metal ions from aqueous solutions with various equilibrium pH values onto pectic acid gel cross linked using formaldehyde [9]. The % adsorption denotes the extent of adsorption and is defined by following equation.
% adsorption = {(initial concentration - concentration after adsorption)/initial concentration} X 100
As seen from this figure, the % adsorption increased with increasing pH, which suggests that adsorption of cationic metal ions takes place by cation exchange between metal ions and hydrogen atoms of carboxylic groups. During adsorption of metal ions by the cation exchange mechanism, two positive charges of the divalent metal ions are neutralized by two carboxylic groups of the acidic polysaccharides as shown in Scheme 4. However, with high-valent metal ions such as trivalent (3) and tetravalent (4), it is difficult for all of positive charges to be neutralized by carboxylic groups because of strong steric hindrance in the acidic polysaccharides. Steric hindrance is particularly problematic in cross linked polysaccharides as it is with the synthetic cation exchange resins discussed earlier. In such cases, the positive charges that are not neutralized by carboxylic groups are neutralized by anionic species (e.g., hydroxyl ions) present in the aqueous solution also similar to the cases mentioned earlier. These anionic species are easily exchanged with other anionic species such as arsenate, arsenite and phosphate.
Adsorption of Metal Ions on Gel Prepared from Orange Juice Residue
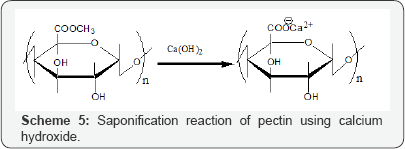

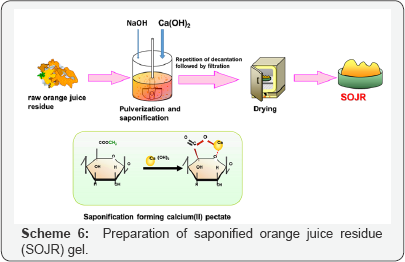
Although cross linked pectic acid gel exhibits good adsorption of metal ions, it is expensive to produce the adsorption gel commercially using pure pectic acid. Consequently, to reduce the cost, we investigated preparation of similar gels from biomass wastes containing large quantities of pectic acid. One candidate biomass wastes was orange juice residue (OJR) [10]. Scheme 6 illustrates preparation of a gel from OJR. Adsorption gels can be easily prepared in a simple and inexpensive manner from the OJR just after juicing. Orange juice is produced from oranges by mechanically peeling and juicing processes. Approximately, half of each orange is left as OJR, which consists of thick outer rind, thin inner peel, seeds and calyces. Currently, OJR is used as cattle feed and fertilizer in Japan. Its main components are polysaccharides such as cellulose and pectin. To prepare the adsorption gel, OJR was treated with calcium hydroxide in a saponification reaction to increase the content of carboxylic acid groups and improve the adsorption capacity for metal ions as shown in Scheme 5. For example, about 100 g of OJR obtained just after juicing was added to a juicer mixer with 8 g calcium hydroxide, and the sample was pulverized. The resulting mixture was transferred into a beaker, along with a large volume of water, and the mixture was stirred for 24 h at 200 rpm and room temperature to facilitate the saponification reaction. The initial pH of the mixture was maintained at around 12.5 by adding sodium hydroxide solution. After decantation, the mixture was repeatedly washed using water and decanted until it reached neutral pH. In this process, water washing is important because OJR contains citric acid, which functions as a water soluble chelating agent, impeding adsorption of metal ions or eluting the loaded metal ions. Finally, the mixture was filtered to obtain a wet gel, which was dried in a convection oven for about 48 h at 700C to obtain dry gel. The prepared dry gel is referred to as saponified OJR (SOJR).An image of the SOJR gel was obtained by scanning electron microscopy as shown in Figure 2. The specific surface area of this gel was measured as 7.25 m2 g-1.
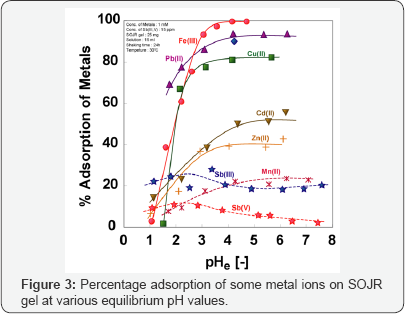
In SOJR gel, pectic acid is present as calcium pectinate, which can be converted into a free acid form, by washing with 0.1 mol dm-3 hydrochloric acid and then water until the pH is neutral. The free acid form of the SOJR gel contained 2.69 mol kg-1 hydrogen ion, while the gel prepared from pure pectic acid gel contained 5.65 mol kg-1 free hydrogen ion. These results suggest that much cheaper adsorbent possessing thesame functional groups with pure pectic acid gel can be produced from biomass waste [10]. Figure 3 shows the adsorption of some metal ions on SOJR gel as plots of % adsorption versus the equilibrium pH values. The adsorption behavior of SOJR gel is similar to that of crosslinked pectic acid gel, and iron(III), lead(II) and copper(II) are selectively and strongly adsorbed on this gel. Iron(III) was found to be adsorbed according to the Langmuir's adsorption model, with a maximum adsorption capacity of 1.55 mol kg-1.The maximum adsorption capacity for iron(III) on cross linked pectic acid gel was 0.57 mol kg-1. This difference in adsorption capacities could be attributed to the effect of calcium(II) contained in the SOJR gel, which we can infer functions as an alkali for iron(III) ion hydrolysis into fine ferric hydroxide particles, that remain in the SOJR gel. Similar adsorption gel was prepared also from apple juice residue [11].
Adsorption of Phosphorus on SOJR Gel Loaded with High-Valent Metal Ions
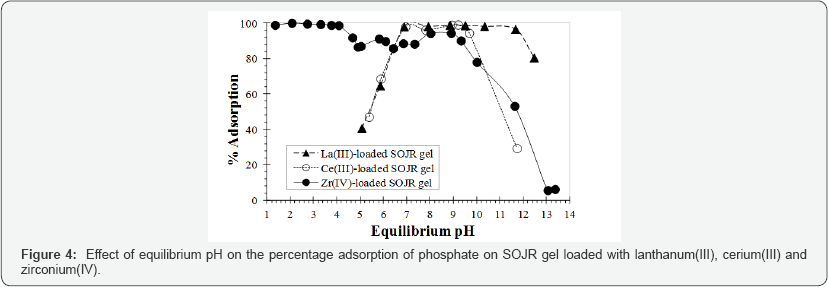
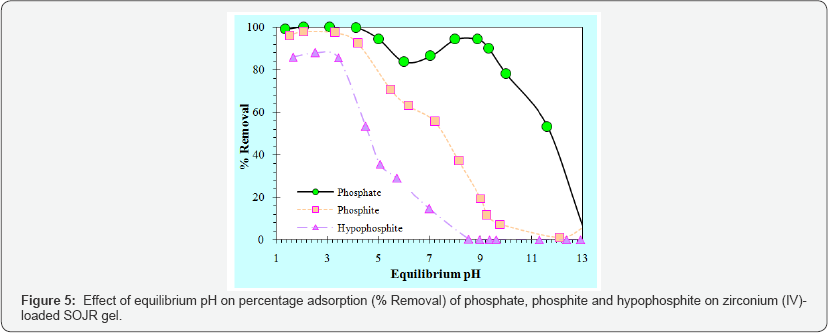
For the removal or recovery of low or trace concentrations of phosphorus from an aquatic environment, the use of SOJR gelpre-loaded with high-valent metal ions was examined as an alternative to synthetic cation exchange resins [12,13]. Figure 4 shows the effect of equilibrium pH on the % adsorption of phosphate (PO43-) on SOJR gel loaded with three high-valent metal ions(lanthanum(III), cerium(III), and zirconium(IV)). As can be seen from this figure, compared with the lanthanum(III)- and cerium(III)-loaded SOJR gels,zirconium(IV)-loaded SOJR gel could adsorb phosphate over a wider pH range, including low pH range (≤6). The decrease in adsorption with increasing pH at values higher than 9 is attributable to exchange of the adsorbed phosphate with hydroxyl ions. Figure 5 shows the effect of equilibrium pH on % adsorption of three anionic species of phosphorus, phosphate (PO43-), phosphite (HPO32-), and hypophosphite (HPO2-), on zirconium(IV)-loaded SOJR gel. It is seen from this figure that the adsorption decreased in the order, phosphate, phosphite and hypophosphite in accordance with their negative charges [14,15].
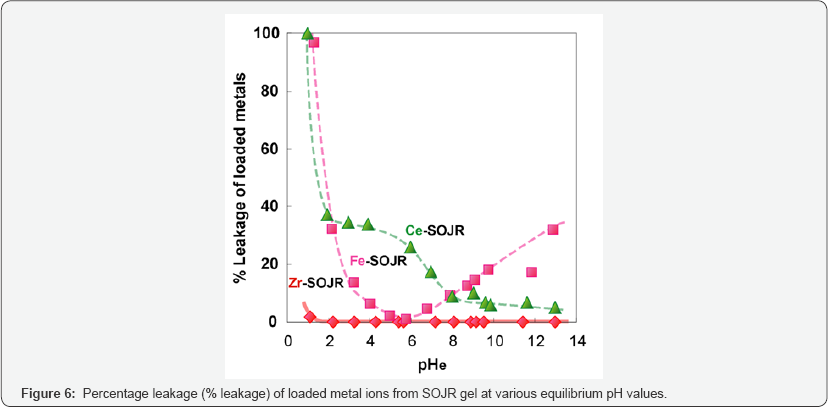
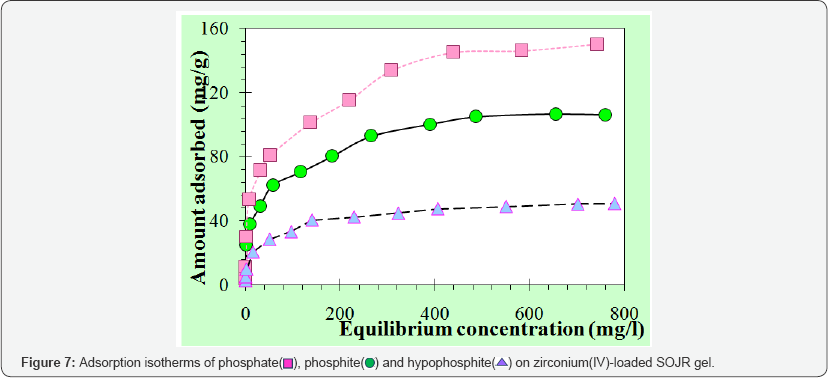
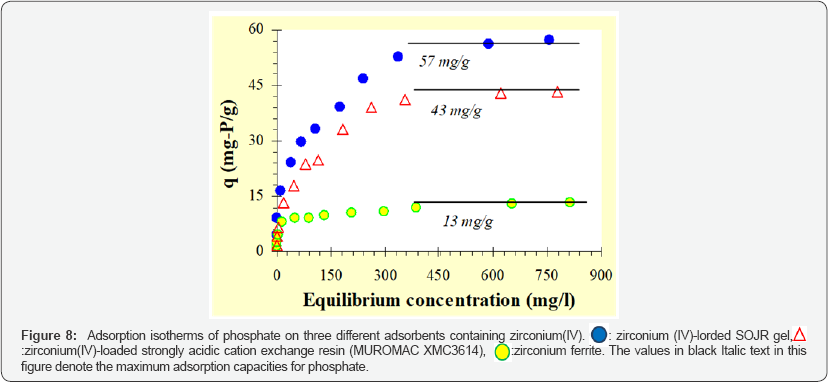
Compared with other metal ions, zirconium(IV) ion has another advantage over other metal ions as the loaded metal ion. Figure 6 shows percentage leakage of the loaded metal ions from the SOJR gel at various equilibrium pH values. As seen from this figure, although some loaded iron(III) and cerium(III) leaked from the SOJR gel at low pH in particular, leakage of zirconium(IV) was negligible. Figure 7 shows adsorption isotherms of phosphate, phosphite and hypophosphite, measured at pH 3, 2 and 2.5, respectively, at 300c on zirconium(IV)-loaded SOJR gel. The plots for these three phosphorus compounds appear to be the Langmuir-type isotherms. From the constant values at plateau region in the high concentration region, the maximum adsorption capacities for phosphate, phosphite and hypophosphite were evaluated as 148, 105 and 50 mg/g dry gel, respectively. Figure 8 shows adsorption isotherms of phosphate at pH around 7 on three different adsorbents containing zirconium(IV). The adsorbents were zirconium(IV)-loaded SOJR gel, zirconium(IV)-loaded strongly acidic cation exchange resin, MUROMAC XMC3614, produced and marketed by Muromachi Chemical Co., Ltd., Omuta, Japan, and zirconium ferrite, a commercially available inorganic adsorbent for phosphorus, the chemical composition of which is ZrFe2(OH)8. All the isotherms were consistent with the Langmuir adsorption model. The maximum adsorption capacities on the adsorbents were also evaluated from the constant values at plateau regions for each adsorbent. It is obvious that zirconium(IV)-loaded SOJR gel had the highest adsorption capacity among these adsorbents. Table 1 compares the maximum adsorption capacities of phosphate on various adsorbents including those reported in literatures. As seen from this table, the zirconium(IV)-loaded SOJR gel had the highest capacity among these adsorbents.
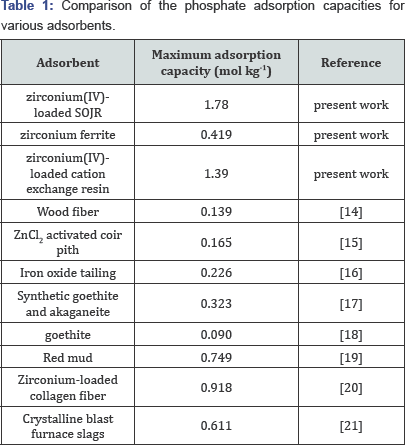
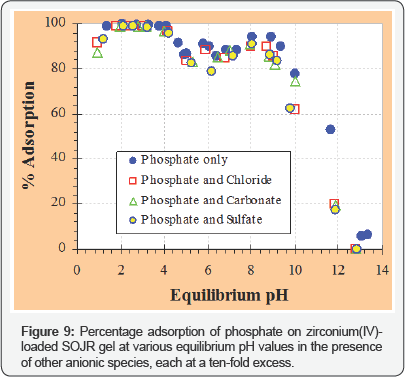
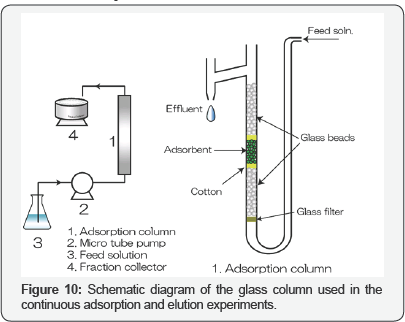
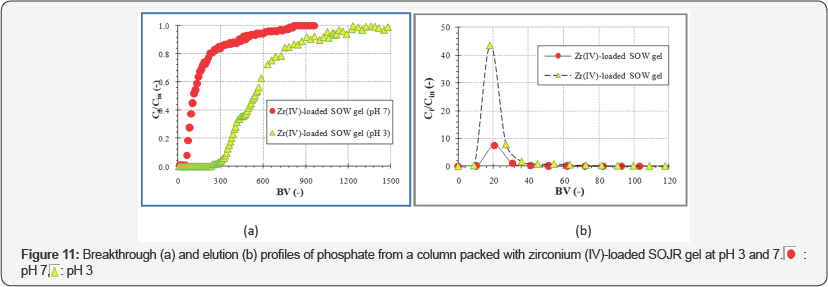
Figure 9 illustrates the plots of percentage adsorption of phosphate on zirconium(IV)-loaded SOJR gel at various equilibrium pH values in the presence of other anionic species, each at a ten-fold excess, to investigate the effect of coexisting anionic species. As seen from this figure, the coexisting anionic species did not influence the adsorption of phosphate. Based on the information about fundamental adsorption behavior of phosphate obtained in batch experiments, tests using continuous adsorption followed by elution were carried out in a glass column shown in Figure 10. Breakthrough profiles of phosphate from a column packed with zirconium(IV)-loaded SOJR gel were measured with one phosphate solution at pH 3 and 7 as shown in Figure 11(a). In this figure, the ordinate denotes the ratio of the outlet concentration at an arbitrary time (Ct) to inlet concentration (C ) and the abscissa denotes the bed volume,which is the ratio of the volume of the feed solution that passes through the column adsorption bed to the volume of adsorbent in the column (i.e. the contact time during the adsorption or elution). As expected from the batch experiments (Figure 4), the breakthrough occurred earlier at pH 7 than at pH 3, that is, more phosphate was adsorbed at pH 3 than at pH 7. Figure 11(b) shows the elution profiles obtained after breakthrough shown in Figure 11(a) using 0.2 mol dm-3 sodium hydroxide solution. After elution, the phosphate concentration was about eight times that in the feed solution when the adsorption was carried at pH 7, and about 44 times at pH3.
To examine the durability of zirconium(IV)-loaded SOJR gel for phosphate solutions, adsorption of phosphate followed by elution using 0.2 mol dm-3 sodium hydroxide solution was repeated up to nine times using the same packed column as shown in Figure 12. As seen in this figure, the adsorption and elution behaviors showed minimal changes in the nine cycles, suggesting this adsorption gel is highly durable for the adsorption of phosphate.
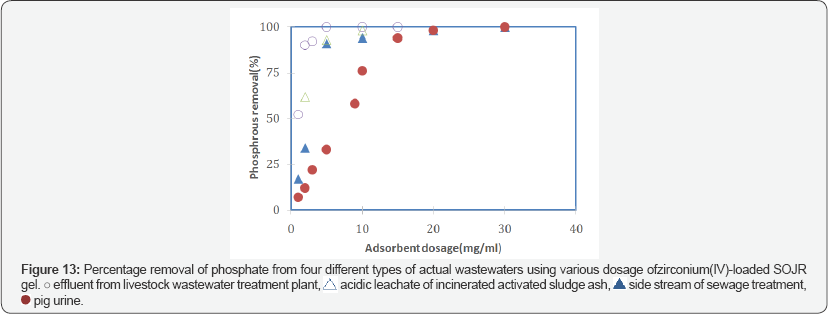
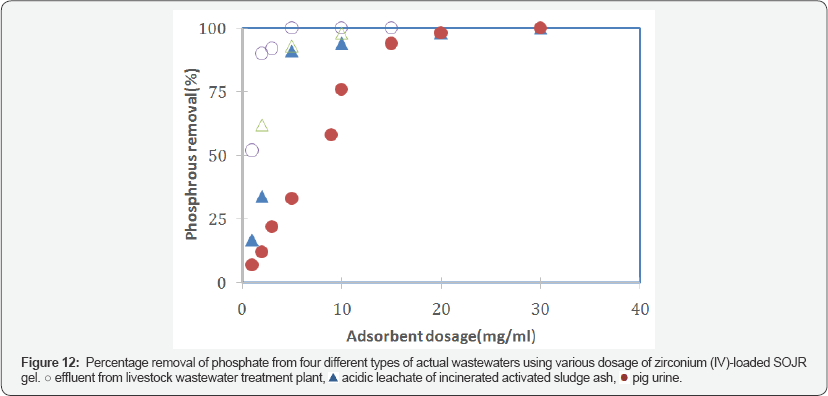
To evaluate the applicability to actual solutions, zirconium(IV)-loaded SOJR gel was tested with actual wastewater samples containing phosphorus. Figure 13 shows the percentage removal of phosphorus using various dosage of zirconium(IV)-loaded SOJR gel from three actual wastewaters containing phosphorus: effluent from livestock wastewater treatment plant, acidic leachate of incinerated activated sludge generated in sewage treatment plant, and pig urine, which had phosphorus content of 38, 367 and 660 mg dm-3, respectively. As seen from this figure, phosphorus could be effectively removed or recovered from each of these actual solutions
Conclusion
An adsorption gel for metal ions was prepared from OJR in a simple and inexpensive manner. Loading high-valent metal ions such as zirconium(IV) on the gel resulted in effective and selective adsorption of trace concentrations of phosphate over other anionic species such as chloride and sulfate. Adsorption and elution of phosphate was successfully controlled by changing the pH. The adsorption capacity for phosphate on this zirconium(IV)-loaded gel was higher than other adsorbents that have been reported in literature, including other zirconium(IV)- containing adsorbents. This gel exhibited high durability for repetitive adsorption followed by elution. The effectiveness of this gel was verified in application to actual waste solutions containing trace concentrations of phosphorus.
Acknowledgement
The present work was financially supported by a Grant-in- Aid for Scientific Research of Waste Treatment from The Ministry of Environment of Japan (K2072, K2164, K22080).
References
- Nowack B, Stone AT (2006) Competitive adsorption of phosphate and phosphonates onto goethite. Wat Res 40(11): 2201-2209.
- WWW.hyobun.co.jp/pdf/kizyn_suisitu.pdf.
- Tanaka T, Shimamura K (2005) Biological wastewater treatment process with chemical-phosphorus recover reactor. J Environ Biotechnol 4(2): 101-108.
- Yoshida I, Ueno K, Kobayashi H (1978) Selective separation of arsenic (III and V) ions with ferric complex of chelating ion-exchange resin. Sep Sci Technol 13: 173-184.
- Dhakal RP, Ghimire KN, Inoue K, Yano M, Makino K (2005) Acidic polysaccharide gels for selective adsorption of lead(ll) ion. Sep Pur Technol 42(3): 219-224.
- Inoue K, Baba Y (2007) Chitosan: A versatile biopolymer for separation, purification, and concentration of metal ions. In Ion Exchange and Solvent Extraction, Sengupta AK (Eds.), CRC Press, Boca Raton, USA 18: 339-374.
- Maruyama T, Matsushita H, Shimada Y, KamataI, Hanaki M, et al. (2007) Proteins and protein-rich biomass as environmentally friendly adsorbents selective for precious metal ions. Env Sci Technol 41: 13591364.
- Kuyucal N, Volesky B (1988) Biosorbents for recovery of metals from in-dustrial solutions. Biotechnol Lett 10: 137-142.
- Inoue K, Shioya A, Zhu Y, Sedlackova I, Makino K, et al. (2003) Adsorptive removal of arsenic by ferric ion-loaded cross linked pectic acid gel. Kagaku Kogaku Ronbunshu 29(3): 389-394.
- Dhakal RP, Ghimire KN, Inoue K (2005) Adsorptive separation of heavy metals from an aquatic environment using orange waste. Hydrometallurgy 79(3-4): 182-190.
- Inoue K, Ghimire KN, Makino K (2003) Removal of impurity metal ions from waste plating solutions by using orange and apple juice residues. ln: Schlesinger ME (Eds.), EPD Congress, The Minerals, Metals & Materials Society (TMS), Warrendale USA, Pp. 525-535.
- Biswas BK, Inoue K, Ghimire KN, Ohta S, Harada H, et al. (2007) The adsorption of phosphate from an aquatic environment using metal- loaded orange waste. J Coll Interface Sci 312(2): 214-223.
- Biswas BK, Inoue K, Ghimire KN, Harada H, Ohto K, et al. (2008) Removal and recovery of phosphorus from water by means of adsorption onto orange waste gel loaded with zirconium. Biores Technol 99(18): 86858690.
- Eberhardt TL, Min SH, Han JS (2006) Phosphate removal by refined aspen wood fiber treated with carboxymethyl cellulose and ferrous chloride. Biores Technol 97(18): 2371-2376.
- Namasivayam C, Sangeetha D (2004) Equilibrium and kinetic studies of adsorption of phosphate onto ZnCl2 activated coir pith carbon. J Coll Interface Sc 280(2): 359-365.
- Zeng L, Li X, Liu J (2004) Adsorptive removal of phosphate from aqueous solutions using iron oxide tailings. Wat Res 38(5): 1318-1326.
- Chitrakar R, Tezuka S, Sonoda A, Sakane K, Ooi K, et al. (2006) Phosphate adsorption on synthetic goethite and akaganeite. J Coll Interface Sci 298(2): 602-608.
- Zhong B, Stanforth R, Wu S, Chen JP(2007) Proton interaction in phosphate adsorption onto goethite. J Coll. lnterface Sci 308(1): 40-48.
- Pradhan J, Das J, Das S, Thakur RS (1998) Adsorption of phosphate from aqueous solution using activated red mud. J Coll Interface Sci 204(1): 169-172.
- Liao XP, Ding Y, Wang B, Shi B (2006) Adsorption behavior of phosphate on metal-ions-loaded collagen fiber. Ind Eng Chem Res 45(11): 38963901.
- Kostura B, Kulveitova H, Lesko J (2005) Blast furnace slags as sorbents of phosphate from water solutions. Wat Res 39(9): 1795-1802.






























