Novel RGO/ ZnW04/Fe304 Nanocomposite as High Performance Electrocatalyst for Oxygen Evolution Reaction in Basic Medium
Mohamed Jaffer Sadiq and Krishna Bhat D*
Department of Chemistry, National Institute of Technology, India
Submission: June 15, 2017; Published: June 29, 2017
*Corresponding author: Krishna Bhat D, Department of Chemistry, National Institute of Technology, Karnataka Surathkal Mangalore-575025, India, Tel: +91 824 2473202; Fax: +91 824 2474033; Email: denthajekb@gmail.com
How to cite this article: Mohamed J S, Krishna B D. Novel RGO/ ZnWO4/Fe3O4 Nanocomposite as High Performance Electrocatalyst for Oxygen Evolution Reaction in Basic Medium. JOJ Material Sci. 2017; 2(2): 555584. DOI:10.19080/JOJMS.2017.02.555584
Abstract
Novel RGO/ZnWO4/Fe3O4 nanocomposites have been synthesized through a facile microwave irradiation method. The prepared nanocomposites were characterized by XPS (X-ray photoelectron spectroscopy), XRD (X-ray diffraction), Raman spectroscopy, FESEM (field emission scanning electron microscopy), TEM (transmission electron microscopy) and HRTEM (high resolution transmission electron microscopy). The electrochemical properties of the fabricated electrodes for the OER (oxygen evolution reaction) in alkaline solution were evaluated by LSV (linear sweep voltammetry) and chrono potentiometry techniques. The results indicate that the ternary nanocomposites have better catalytic activity in the OER process than component materials. Interestingly, the ternary nanocomposite shows small onset potential 0.619V, small Tafel slope 90mV/dec, high current density 6.65mA/cm2 and highly stable even after 1000 OER cycles. Hence, the as prepared nanocomposites are cost effective and can function as highly efficient noble metal free electrocatalysts for OER applications.
Keywords: Oxygen evolution reaction; Linear sweep voltammetry; Electrocatalyst; RGO/ZnWO4/Fe3O4 nanocomposites; Microwave irradiation method
Introduction
Normally, growing energy demands provide opportunities for the development of new technologies and efficient materials for electrochemical energy conversion and storage devices [1]. With increasing energy consumption and the associated environmental problems, finding a sustainable and clean energy source has become the need of the day in the present world [2]. Water splitting technology to produce pure hydrogen has been investigated for many years as an avenue for clean energy [3]. A promising way to produce hydrogen is through electrolysis of water, which is composed of two half-cell reactions - HER (hydrogen evolution reaction) at the cathode and OER (oxygen evolution reaction) at the anode [4]. In the water splitting reaction, there is a high over potential at the anode, due to the sluggish four electron transfer oxygen evolution kinetics. This has to be addressed to produce pure hydrogen in a facile way [5]. Thus, in order to accelerate the reaction rate and lower the anode over potential, it is essential to put in great efforts to find efficient electrocatalysts for OER [6]. The most extensively investigated metal catalysts such as RuO2, IrO2, and Pt/C were proved to be the promising candidates as catalysts for OER [7], Despite their good catalytic activity, they are expensive and have scarce availability which has severely restricted the large scale commercial applications of these materials [8]. Therefore, it is necessary to develop high-performance, cost effective, ecofriendly and noble metal free OER catalysts which are more-efficient, stable and earth-abundant. Various materials based on first row transition metals have proved to be efficient OER catalysts [9,10]. Graphene exhibits fascinating physical and chemical properties, such as excellent conductivity, high surface area and extraordinary electrocatalytic activities [11]. Therefore, it has been used as a suitable supporting material for OER catalysis [12]. Recently we published the synthesis of novel graphene - zinc tungstate - magnatite nanocomposite as high performance catalyst for photodegradation and reduction of 4-nitrophenol [13,14]. Inspired by the encouraging results obtained in those studies, we report herein, investigation on the catalytic activity of the novel RGO/ZnWO4/Fe3O4 nanocomposites for OER. The experimental results indicate that these novel ternary nanocomposites show a great promise as future noble metal free catalysts for OER in basic medium.
Experimental
Material synthesis
All the chemicals were of analytical grade and were purchased from Sigma Aldrich. All the solutions used in the study were prepared from Millipore water. GO (Graphene oxide) was synthesized from graphite flakes via modified Hummers method [15]. RGO/ZnWO4/Fe3O4 nanocomposite was synthesized by one- step microwave irradiation method. Required amount of GO was dispersed into 50mL ethylene glycol using ultrasonic treatment. To this 50mL each of 0.05 M of zinc acetate and sodium tung state solution was added slowly under stirring at the pH of 9 (maintained using ammonia) for about 2 hours. Later, the above mixture was irradiated with microwave radiation at 350W for 10 minutes and obtained RGO/ZnWO4 nanocomposite. To the cooled solution, 50mL of iron acetate (0.01M) and 10mL of ammonia solution was added under stirring for about 30 minutes after which it was irradiated with microwave radiation (350W) for 10 minutes. The obtained RGO/ZnWO4/Fe3O4 precipitate was washed with (90:10) water and ethanol several times. Finally, the sample was dried in a vacuum chamber at 80 °C for 12 hours. The procedure was repeated without the use of one of the component for the sake of comparison.
Characterization
To determine the elemental composition of the sample, XPS was performed (Multilab 2000, Thermo scientific, UK) using Mg- Ka X-ray (1253.6eV) with 200W power as exciting source and 10eV energy pass for data collection. The crystal structure was determined by XRD (Rigaku Corporation, Japan) analysis using nickel-filtered Cu-Ka radiation (=1.5406 A). Raman spectra were recorded by laser Raman microscope (Renishaw) with 532nm He-Ne excitation source. The morphology of the samples was investigated by FESEM (Zeiss Ultra 55), TEM and HRTEM (Tecnai).
Electrochemical measurements
In a typical experiment, 1.0mg of the catalyst was well dispersed in 495μL of water and 5μL of 5wt. % Nafion by using ultrasonic treatment for 20 minutes to form homogeneous paste. Then, 3.0μL of the paste was drop cast on GC (glassy carbon) electrode (diameter of 3mm) surface and dried at room temperature. Electrochemical experiments for OER were performed using an IVIUM instrument in a standard three- electrode system. A GC, saturated calomel electrode (SCE) and platinum wire served as working, reference and counter electrode respectively
Results and Discussion
XPS analysis
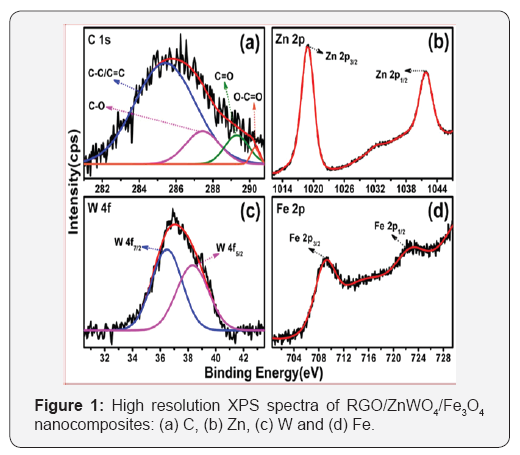
The XPS spectrum of the as-prepared RGO/ZnWO4/Fe3O4 nanocomposite is shown in Figure 1 & 1a illustrates the C 1s spectral region, which could be de convoluted into four peaks with binding energies of 285eV, 287.1eV, 288.5eV and 290.3eV.
These peaks are assigned to sp2 C-C/C=C bonds in the aromatic ring, C-O, C=O and O-C=O bonds in the oxygenated functional groups respectively which indicates that GO has been reduced to graphene sheets [16-21].Figure 1b displays the Zn 2p region, composed of two peaks at 1021.5eV and 1044eV which corresponds to the Zn 2p3/2 and Zn 2p1/2 state, respectively. Figure 1c depicts the W 4f region, consisting of two peaks at 35.7 and 38eV are assigned to W 4f7/2 and W 4f5/2. These results are consistent with the previously reported values for ZnWO4 [22]. Figure 1d portrays binding energies of Fe 2p region, two peaks are found at 710.8eV and 724.5eV corresponding to Fe 2p3/2 and Fe 2p1/2, indicating that Fe3O4 particles [23]. The above results show that ZnWO4 and Fe3O4 particles are well decorated on RGO sheets successfully.
XRD analysis
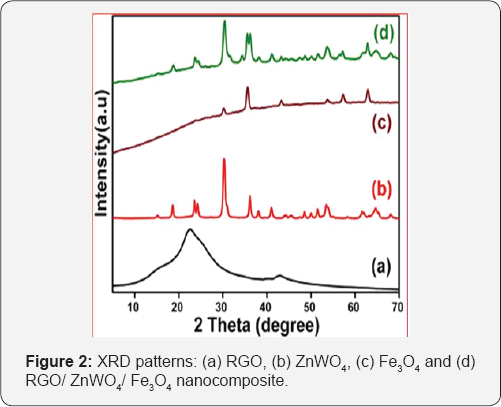
The crystal structure of the materials was evaluated via X-ray diffraction techniques. Figure 2 shows the XRD patterns of RGO, ZnWO4, Fe3O4 and RGO/ZnWO4/Fe3O4 nanocomposite. The observed broad peaks around at 22.6° and 42.6° in Figure 2a can be well indexed to the (002) and (100) planes of RGO nanosheets, respectively. Figure 2b shows the XRD traces of ZnWO4. The diffraction peaks at 15.3°, 18.7°, 23.6°, 24.3°, 30.4°, 36.3°, 38.2°, 41.0°, 44.4°, 45.5°, 48.6°, 50.1°, 51.5°, 53.9°, 61.7°, 64.9° and 68.1° can be well indexed to the (010), (100), (011), (110), (111), (021), (200), (121), (112), (211), (022), (220), (130), (122), (113), (311) and (041) crystalline planes of ZnWO4 (JCPDS 15-0774), respectively. The diffraction peaks at 30.3°, 35.6°, 43.4°, 53.7°, 57.3° and 62.9° as observed in Figure 2c can be well indexed to (220), (311), (400), (422), (511) and (440) crystalline planes of Fe3O4 (JCPDS 19-0629), respectively. Figure 2d shows the XRD pattern for the nanocomposite, RGO/ZnWO4/ Fe3O4. As can be seen from the figure, all the crystalline planes corresponding to ZnWO4 and Fe3O4 can be identified. The RGO peaks are not visible clearly because of the very small amount of the material present in the composite. The results confirm the presence of crystalline ZnWO4 and Fe3O4 being incorporated on the RGO nanosheets in the RGO/ZnWO4/Fe3O4 nanocomposite.
Raman analysis
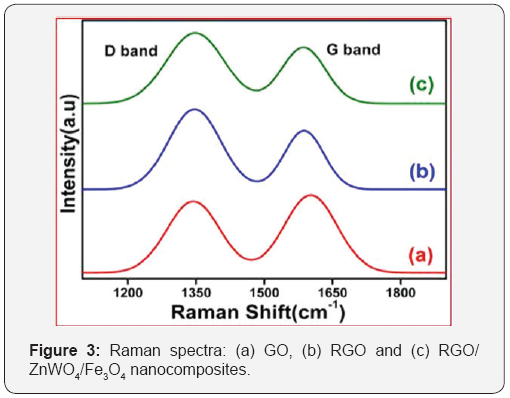
Figure 3 shows the further structural information on the as-synthesized RGO/ZnWO4/Fe3O4 nanocomposites as found from Raman spectroscopy. It is observed that, the D and G band peaks of GO appear at 1349cm-1 and 1604cm-1, respectively with the measured (ID/IG) Intensity ratio being equal to 0.98 (Figure 3a). As shown in Figure 3b, the same for RGO are found at 1347cm-1 and 1600cm-1 and measured (ID/IG) Intensity ratio is 1.10. The variation of (ID/IG) Intensity ratio from GO to RGO is related to the elimination of functional groups and formation of defects along with the recovery of sp2 conjugated carbon structure during the reduction of GO into RGO nanosheets [16,21]. The Raman spectra of RGO/ZnWO4/Fe3O4 nanocomposite (Figure 3c) exhibits the D and G bands at 1348cm"1 and 1601cm"1 respectively. The measured (ID/IG) Intensity ratio is 1.03, which is slightly lower than the RGO nanosheets. This decrease in ratio is attributed to the non-covalent interactions of nanoparticles on the RGO nanosheets [23].
Morphology analysis
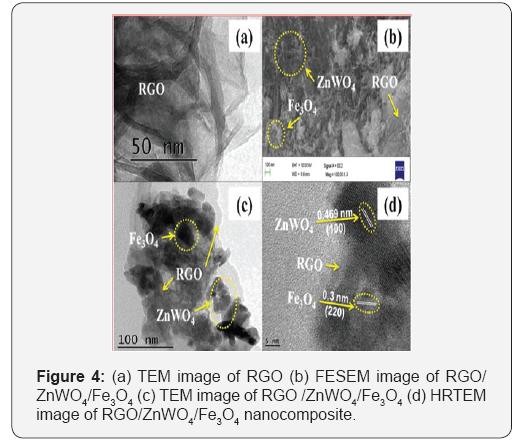
The structural morphology of the materials was estimated via electron microscopy techniques. Figure 4a clearly shows the transparent RGO nanosheets with thin, crumpled and folded structures. Figure 4b shows the FESEM image of the RGO/ ZnWO4/Fe3O4 nanocomposite and Figure 4c is TEM image of the same wherein the rod shaped ZnWO4 and spherical like Fe3O4 nanomaterial being anchored on the surface of the RGO nano sheets can be observed. The presence of ZnWO4 and Fe3O4 nanomaterials in the nanocomposite is further confirmed by HRTEM analysis shown in Figure 4d. The observed lattice fringes of 0.469nm and 0.3nm correspond to the (100) and (220) planes of the ZnWO4 and Fe3O4 respectively [24].
Electrochemical analysis
To evaluate the electrocatalytic behavior of RGO/ ZnWO4/ Fe3O4 towards OER, LSVs were carried out at 0.1M KOH with scan rate of 5mV/s in a three-electrode system. Figure 5a shows that the onset potential of OER detected for the RGO/ZnWO4/ Fe3O4 nanocomposite (0.619V) is least compared to that for RGO/ Fe3O4 (0.669V), RGO/ZnWO4 (0.701V), ZnWO4 (0.726V) and RGO (0.752V). The corresponding voltammetric current density values for these materials are 6.65mA/cm2, 3.65mA/cm2, 1.79mA/cm2, 0.98mA/cm2 and 0.59mA/cm2 respectively. Figure 5b shows the kinetics behavior for the ZnWO4, RGO/ZnWO4, RGO/Fe3O4 and ZnWO4-RGO-Fe3O4 nanocomposites as estimated by using Tafel slopes. The linear region of the Tafel plots were fitted with the Tafel equation [17] (n = b log j + a, where b is the Tafel slope, j is the current density, n is the overpotential and a is the constant). The calculated Tafel slopes are 138mV/dec, 120mV/dec, 102mV/dec and 90mV/dec for ZnWO4, RGO/ZnWO4, RGO/Fe3O4 and RGO/ZnWO4/Fe3O4 composite, respectively The small onset potential, small Tafel slope and higher current density observed for the ternary composite indicate its higher efficiency towards OER. The observed results suggest that the RGO/ZnWO4/Fe3O4 nanocomposite possesses good catalytic activity towards OER with small over potential, small Tafel slope and high current response. Also, it may also be noted that although the activity this material is slightly lower compared to that of the benchmark Ru/C & Ir/C based materials, the present nanocomposite has the advantage of easy availability, facile synthesis and low cost. Further, the stability and reusability of the synthesized material has also been tested. Figure 5c shows the stability test for RGO/ZnWO4/Fe3O4 nanocomposites. The small negative shift in current density even after 1000 cycles indicates the high stability and reusability of RGO/ZnWO4/Fe3O4 nanocomposite and indicates that it can function as an efficient OER catalyst for commercial applications [25,26].
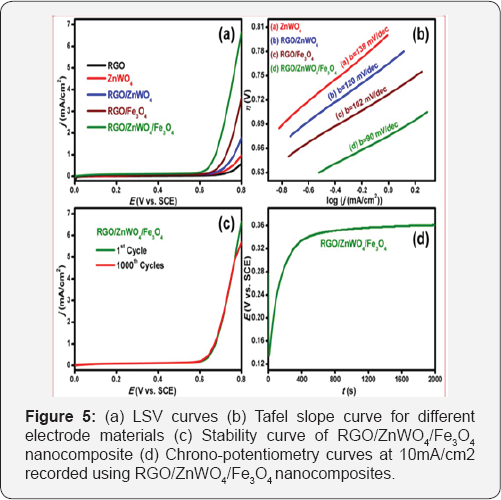
Further, the commercial application of the electrocatalyst has been studied by chrono potentiometry technique at constant current density applied over sufficient period oftime (t). Figure 5d shows that the chrono potentiometry study of the RGO/ZnWO4/ Fe3O4 nanocomposite at a constant current density of 10mA/cm2 for duration of 2000 seconds. As can be observed from the figure, initially with time the potential (E) increased rapidly up to 400 seconds and afterwards the value slowly reached saturation suggesting the establishment of the stabilized state of OER. This phenomenon is attributed to the development of O2 bubbles on the electrode surfaces. Overall, the results demonstrate that the RGO/ZnWO4/Fe3O4 nanocomposites are indeed highly efficient electrocatalysts for OER in basic medium [27].
Conclusion
In summary, a facile microwave irradiation method has been used to synthesize novel RGO/ZnWO4/Fe3O4 naocomposite. The RGO/ZnWO4/Fe3O4 nanocomposite exhibits high electrocatalytic activity for OER in 0.1M KOH solution with small onset potential of 0.619 V, small Tafel slope of 90mV/dec and high current density of 6.65mA/cm2. Further, the catalyst also shows high stability and efficiency even after 1000 cycles. The method developed by this study could be used for large scale synthesis of noble metal free high performance electrocatalyst for OER applications.
Acknowledgment
M.J.S.M. is grateful to NITK Surathkal for the award of a research fellowship.
References
- Bard AJ, Fox MA (1995) Artificial photosynthesis: solar splitting of water to hydrogen and oxygen. Acc Chem Res 28(3): 141-145.
- Park S, Shao Y, Liu J, Wang Y (2012) Oxygen electrocatalysts for water electrolyzers and reversible fuel cells: status and perspective. Energy Environ Sci 5(11): 9331-9344.
- Turner JA (2004) Sustainable hydrogen production. Science 305(5686): 972-974.
- Meyer TJ (2008) Catalysis: The art of splitting water. Nature 451(7180): 778-779.
- Li P, Zhao X, Jia CJ, Sun H, Sun L, et al. (2013) ZnWO4/BiOI heterostructures with highly efficient visible light photocatalytic activity: the case of interface lattice and energy level match. J Mater Chem A 1(10): 3421-3429.
- Bajdich M, Garci'a-Mota MN, Vojvodic A, N0rskov JK, Bell AT (2013) Theoretical investigation of the activity of cobalt oxides for the electrochemical oxidation of water. J Am Chem Soc 135(36): 1352113530.
- Kadakia K, Datta MK, Velikokhatnyi OI, Jampani P, Park SK, et al. (2014) High performance fluorine doped (Sn, Ru) O2 oxygen evolution reaction electro-catalysts for proton exchange membrane based water electrolysis. J Power Sources 245: 362-370.
- Bian W, Yang Z, Strasser P, Yang R (2014) A CoFe2O4/graphene nano hybrid as an efficient bi-functional electrocatalyst for oxygen reduction and oxygen evolution. J Power Sources 250: 196-203.
- Burke MS, Enman LJ, Batchellor AS, Zou S, Boettcher SW (2015) Oxygen Evolution Reaction Electro catalysis on Transition Metal Oxides and (Oxy) hydroxides: Activity Trends and Design Principles. Chem Mater 27(22): 7549-7558.
- Deng X, Tuysuz H (2014) Cobalt-Oxide-Based Materials as Water Oxidation Catalyst: Recent Progress and Challenges. ACS Catal 4(10): 3701-3714.
- Chen S, Duan J, Han W, Qiao SZ (2014) A graphene-MnO2 framework as a new generation of three-dimensional oxygen evolution promoter. Chem Commun 50(2): 207-209.
- Suryanto BH, Lu X, Zhao C (2013) Layer-by-layer assembly of transparent amorphous Co3O4 nanoparticles/graphene composite electrodes for sustained oxygen evolution reaction. J Mater Chem A 1(41): 12726-12731.
- Sadiq MMJ, Shenoy US, Bhat DK (2016) Novel RGO-ZnWO4-Fe3O4 nanocomposite as high performance visible light photocatalyst. RSC Adv 6(66): 61821-61829.
- Sadiq MMJ, Bhat DK (2016) Novel RGO-ZnWO4-Fe3O4 Nanocomposite as an Efficient Catalyst for Rapid Reduction of 4-Nitrophenol to 4-Aminophenol. Ind Eng Chem Res 55(27): 7267-7272.
- Hummers WS, Offeman RE (1958) Preparation of graphitic oxide. J Am Chem Soc 80(6): 1339-1339.
- Sadiq MMJ, Mutyala S, Mathiyarasu J, Bhat DK, (2017) RGO/ZnWO4/ Fe3O4 nanocomposite as an efficient electrocatalyst for oxygen reduction reaction. Journal of Electroanalytical Chemistry 799: 102110.
- Sadiq MMJ, Shenoy US, Bhat DK, (2017) NiWO4-ZnO-NRGO ternary nanocomposite as an efficient photocatalyst for degradation of methylene blue and reduction of 4-nitrophenol. Journal of Physics and Chemistry of Solids 109: 124-133.
- Sadiq MMJ, Bhat DK, (2017) A facile microwave approach to synthesize RGO-BaWO4 composites for high performance visible light induced photocatalytic degradation of dyes. AIMS Mater Sci 4(2): 487-502.
- Sadiq MMJ, Bhat DK (2017) Novel ZnWO4/RGO nanocomposite as high performance photocatalyst. AIMS Mater Sci 4(1): 158-171.
- Sadiq MMJ, Shenoy US, Bhat DK, (2017) Enhanced photocatalytic performance of N-doped RGO-FeWO4/Fe3O4 ternary nanocomposite in environmental applications. Mat Today Chem 4: 133-141.
- Sadiq MMJ, Shenoy US, Bhat DK, (2017) High performance dual catalytic activity of Novel zinc tungstate - reduced graphene oxide nanocomposites. Advanced Science Engineering and Medicine 9: 1-7.
- Liang Y, Li Y, Wang H, Dai H (2013) Strongly coupled inorganic/ nanocarbon hybrid materials for advanced electrocatalysis. J Am Chem Soc 135(6): 2013-2036.
- Cheng L, Zhang S, Wang Y, Ding G, Jiao Z (2016) Ternary P25- graphene-Fe3O4 nanocomposite as a magnetically recyclable hybrid for photodegradation of dyes. Mater Res Bull 73: 77-83.
- Wang D, Pan Z, Wu Z, Wang Z, Liu Z (2014) Hydrothermal synthesis of MoS2 nanoflowers as highly efficient hydrogen evolution reaction catalysts. J Power Sources 264: 229-234.
- Lu Z, Xu W, Zhu W, Yang Q, Lei X, et al. (2014) Three-dimensional NiFe layered double hydroxide film for high-efficiency oxygen evolution reaction. Chem Commun 50(49): 6479-6482.
- Reier T, Oezaslan M, Strasser P (2012) Electrocatalytic Oxygen Evolution Reaction (OER) on Ru, Ir, and Pt Catalysts: A Comparative Study of Nanoparticles and Bulk Materials. ACS Catal 2(8): 1765-1772.
- Zhang G, Lu W, Cao F, Xiao Z, Zheng X (2016) N-doped graphene coupled with Co-nanoparticles as an efficient electrocatalyst for oxygen reduction in alkaline media. J Power Sources 302: 114-125.






























