Influence of Annealing Temperature on Co0.4Mg0.6Fe2O4 Nano-Ferrite
H S Mund*
Hutatma Rajguru Mahavidyalaya Arts, Science & Commerce, India
Submission: May 07, 2017; Published: June 29, 2017
*Corresponding author: H S Mund, Rajguru Mahavidyalaya, Shrerawatpura, Jaipur, Rajasthan-303 706, India, Email: hmoond@gmail.com
How to cite this article: H S Mund. Influence of Annealing Temperature on Co0.4Mg0.6Fe2O4 Nano-Ferrite. JOJ Material Sci. 2017; 2(2): 555581. DOI:10.19080/JOJMS.2017.02.555581
Abstract
In this paper, we have reported the effect of annealing temperature on structural properties of Co0.4Mg0.6Fe2O4 nano-ferrites prepared via self-combustion method without using any external oxidizing agents to change the pH of the precursors. The self-combustion method offered homogenous particle size over the composition at a lower temperature. X-ray diffraction (XRD) studies show that particle size was increased from 15nm to 59nm with increasing annealing temperature for Co-Mg nano-ferrites. The spinel ferrite functional group formation was identified by the analysis of FTIR spectroscopy data. Raman spectroscopy was used to study the structural stability of nano-ferrite.
Keywords: X-ray diffraction; Raman spectroscopy; Nano-magnetic properties; Dielectric constant
Introduction
In recent decay, there is a great interest has been taken on the experimental and theoretical research on the nano-ferrites due to their unique and unusual physical and chemical properties which is useful in various technological applications such as switching circuits, high density magnetic storage, microwave based instruments, telecommunication equipment, magnetic fluids, gas sensors, etc. [1-5]. It is established that structural, electric and magnetic properties of nano-ferrite depend on the synthesis method, pH, type and nature of mixing, sintering temperature, cation distribution between tetrahedral and octahedral sites. Although plentiful reports are available on the structural, magnetic and electric response of Mg-doped cobalt ferrites, but work related to systematic study of the effect of annealing temperature on Co0.4Mg0.6Fe2O4 ferrites prepared via self-combustion method is still lacking. Therefore, in the present paper, we have endeavored to investigate the effect of annealing temperature on structural properties of Co0.4Mg0.6Fe2O4 nanoferrite.
Experimental
Co0.4Mg0.6Fe2O4 nano-ferrite was synthesized by the self- combution method using a mixture of metal nitrates with citric acid. The molar ratio of nitrates to citric acid was 1:1. The stoichiometric amount of metal nitrates and citric acid were dissolved in 50ml deionized water separately, mixed and stirred to get a homogenous solution. The homogenous solution was continually stirred with a hot plate at 100 °C to get the liquid gel. The liquid gel was heated on a hot plate until it burnt out completely in the powder form. The obtained powder was grounded and sintered at different temperature i.e. 200, 400, 600, 800 and 1000 °C for 6h. The phase formation and structural properties were carried out using Rigaku MiniFlex X-ray Diffracto meter with Cu-Kα radiation. The XRD patterns were measured over the 2θ range from 20-80° with a step size of 0.02°. Fourier transform infrared (FTIR) spectra were recorded in the frequency range 400-4000cm-1 using PerkinElmer spectrometer. The Raman spectra of these ferrites were observed using STR- 500 Confocal micro-Raman spectrometer.
Results and Discussion
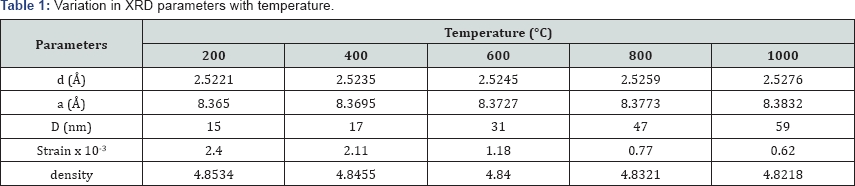
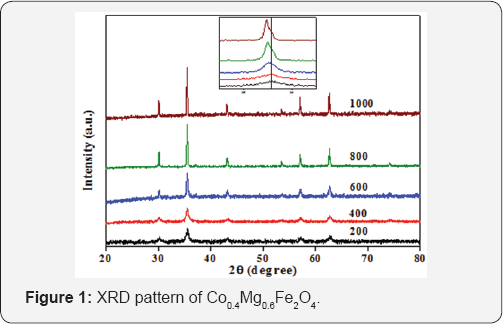
The XRD pattern of Co0.4Mg0.6Fe2O4 nano-ferrites annealed at different temperature i.e. 200, 400, 600, 800 and 1000 °C are shown in Figure 1. XRD pattern analysis shows that all the ferrites have single phase cubic spinel structure without any impurity peak belonging to the space group Fd3m. All the characteristic peaks are in good agreement with the standard JCPDS card No 22-1086. Insets of Figure 1 show that 2θ shifts towards the lower angle side as increasing the annealing temperature for both the ferrites. As annealing temperature increases the intensity of (311) peak become higher while peak broadening becomes narrower,which indicates an increase in the crystallinity and particle size. The average particle size (D) of these ferrites is calculated using the Debye-Scherrer's formula. The specific surface area was calculated assuming all the particles in spherical form using the Sauter formula. The crystallite size, specific surface area, lattice constant, X-ray density and micro strain of all the samples are listed in Table 1. The variation in crystalline size and X-ray density as a function of annealed temperature are shown in Figure 2. The XRD results indicate the formation of crystallinity with increasing the calcination temperature.
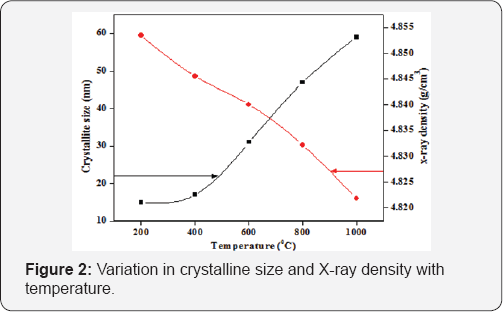
The formation of cubic phase spinel structure in cobalt ferrites was confirmed by FTIR spectra. (Figure 3a) shows the IR spectra in the wave number range of 4000-400cm-1. The FTIR spectrum shows that in ferrites, the metal ions occupy two different interstitial sites in the lattice; one is at the tetrahedral site while the other is at the octahedral site according to the configuration of the oxygen nearest neighbors in fcc structure [6]. The two principle absorption band in the range of 600- 400cm-1 are observed in IR spectra where the high-frequency band observed between 580-578cm'1 for all samples has been attributed to stretching vibrations of tetrahedral metal-oxygen bonding while the lower frequency band observed in between the frequency range 424-407cm-1 due to the octahedral metal- oxygen vibrations. A very week band near 1380cm-1 is found due to the nitrate group which remained as a residue in the sample while bands observed near 1600cm-1 correspond to the stretching and bending modes of H-O-H due to the moisture or absorbed water during sample preparation process.
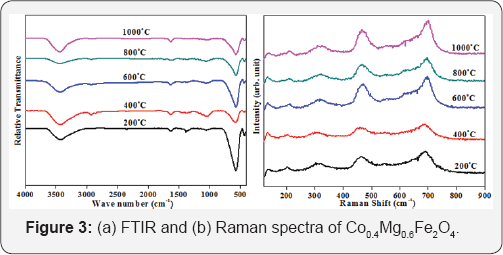
The Raman spectroscopy technique is a powerful and sensitive tool to gain insight into the vibrational energy states and structural information of the compound. Figure 3(b) shows the Raman spectra in the wave number range of 180-900cm'1. Factor group analysis predicts that a cubic phase inverse spinel structure with space group Fd-3m having five Raman active modes namely A1g +Eg+3T2g. In these active modes, A1g mode is due to the symmetric stretching of metal oxygen (Co-O and Fe-O) bonds along the tetrahedral site, while the E mode is a result of the symmetric bending of an oxygen atom with the metal ions [7,8]. The T2g (2) modes rise due to the asymmetric stretching of metal-oxygen bonds (Co/Fe-O) along the octahedral site, while T2g (3) modes are due to asymmetric bending of metal-oxygen bonds of the octahedral site. The T2g (1) mode is attributed due to the translational movement of the metal ion at the tetrahedral site together with four oxygen atoms. The active modes of Raman spectra in between the region 660-720cm-1 is indicative of tetrahedral site, while modes in between 460-640cm-1 are indicative of the octahedral site [8]. The ionic radii of cobalt and iron are different which occur a considerable difference in Co/Fe-O bond length. This distribution in bond length creates a doublet like peak where one peak corresponds to the cobalt ions at octahedral site, while another peak represents the occupancies of cobalt ions both octahedral as well as tetrahedral sites. This doublet like behavior of Raman spectra shows the mixed spinel structure of cobalt ferrites, which is a confirmation of XRD and FTIR results. Thus it is found that structure remains identical, while XRD parameters alteration with increasing annealing temperature.
Acknowledgment
Author is thankful to Head, Department of Physics, M. L. Sukhadia University, Udaipur for XRD measurement under DST- FIST program and Material Research Centre, MNIT, Jaipur for providing FTIR and Raman facility.
References
- Satyanarayana L (2003) Mater Chem Phys 82: 21.
- Pedro T, Marfa del PM, Sabino VV, Teresita GC, Carlos JS (2003) The preparation of magnetic nanoparticles for applications in biomedicine. J Phys D Appl Phys 36: R-182.
- Qu Y (2006) Mater Lett 60: 3548.
- Rana S, Gallo A, Srivastava RS, Misra RD (2007) On the suitability of nanocrystalline ferrites as a magnetic carrier for drug delivery: functionalization, conjugation and drug release kinetics. Acta Biomater 3(2): 233-242.
- Zi Z (2009) J Magn Magn Mater 321: 1251.
- Brabers VAM (1969) Infrared Spectra and Cation Distributions of Manganese Ferrites. Phys Status Solidi 33: 563.
- Modi KB (2006) Ceram Int 32: 111.
- Waldron RD (1955) Infrared Spectra of Ferrites. Phys Res 99: 1727.






























