Effect of Annealing on the Preparation of CuWO4 Particles
Kannan S1, Mohanraj K1* and Siva Kumar G2
1Department of Physics, Manonmaniam Sundaranar University, India
2Department of Physics, Annamalai University, India
Submission: April 29, 2017; Published: June 23, 2017
*Corresponding author: Mohanraj K, Department of Physics, Manonmaniam Sundaranar University, Tirunelveli-627012, Tamilnadu, India Email: kmohanraj.msu@gmail.com
How to cite this article: Kannan S, Mohanraj K, Siva K G. Effect of Annealing on the Preparation of CuWO4 Particles. JOJ Material Sci.2017; 2(1): 555577. DOI:10.19080/JOJMS.2017.02.555577
Abstract
The article compare the conventional annealing (CA-500 °C and 800 °C) and hybrid microwave annealing (HMA-360s) on CuWO4 particles with varies the solution pH values (3 and 9). It is observe that the CA yield high crystalline Copper tungstate (CuWO4) with CuO, while the HMA showed impurity free CuWO4 nanoparticles.
Keywords: Nanoparticles; Polyol-hydrothermal process; Oxidation
Introduction
CuWO4 is an important semiconductor and used for lithium ion battery [1], NO2 gas sensor [2], water splitting [3], photo catalytic degradation [4] and super capacitor [5]. The W-rich and Cu-rich CuWO4 were also prepared by the hydrothermal process, the furthest one showed more photo catalytic activity than the other and pure CuWO4 nanoparticles [6]. Generally, annealing was focused for solid state reaction, phase transformation, removal of water or additional element and oxidation etc. The annealing process can be performed through either conventional or microwave method. In this work, HMA was adopted, due to drastic reduction of processing time and low energy consumption, for the preparation of CuWO4 particles by polyol-hydrothermal process. For the sake of comparison, the CA also used.
Experimental Procedure
0.5M Na2WO4.2H2O solution was added drop by drop into 0.5M Cu(NO3)2.3H2O solution under stirring followed by the addition of 0.3M of polyethylene glycol (Mw=4000). Sky blue colour of precipitate was drown into 50mL Teflon linked autoclave and subjected into heat at 180 °C for 24h. Then, the product was centrifuged using DW and ethanol. The yellowish green colour product was filtered and dried in oven at 80 °C for 8h. The as-prepared product was annealed in two ways: one was conventional method (for 500 °C and 800 °C) and another was HMA using microwave oven (2.45GHz, 800W) for 360s. Graphite powder was used as susceptor. The prepared samples were characterised by using XRD (X’pert Pro diffracto meter with Cu Ka radiation (λ=1.5406 Å) in the range of 10°-80°) and FTIR spectrum were recorded in the KBr technique using Perkin Elmer spectrometer (Spectrum Two, Model: C92107) with resolution of 4cm-1 analyses.
Results and Discussion
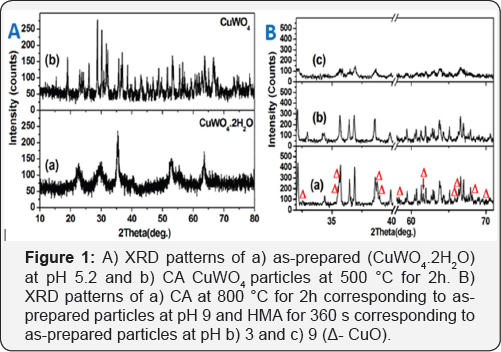
In Figure 1A(a), the as-prepared particles shows amorphous in nature and the 2θ values depicts monoclinic system of wolframite structure CuWO4•2H2O (JCPDS card: 33-0503. In the CA at 500 °C for 2h (Figure 1A(b)), number of sharp and crystalline peaks observed indicates the formation of triclinic CuWO4 alone (JCPDS card: 73-1823). When increasing annealing temperature to 800 °C for 2h (Figure 1B(a)), the XRD pattern shows more crystalline peaks of CuWO4 with CuO (JCPDS card: 89-5895). Similar result was reported by Chen and Xu (2015). According to their investigation WO3 was formed along with CuWO4 at pH ≤3 and CuO was formed at high pH (~8.2) [6]. However, their intensity of crystalline peaks decreased with increase of pH value in HMA samples. By comparing the results with CA, no additional phases such as WO3 and CuO are formed in HMA samples Average crystallite size is found to be around 102.8nm for CA as calculated from the standard Scherer equation. It is considerably reduced as 39.43nm for pH 3 and 97.43nm for pH 9 in HMA. In the FTIR spectrum of as- synthesized particles (Figure 2), a number of vibrational bands are shown at 896, 795, 730, 584, 510 and 367cm-1 belongs to structural and bending vibrations of CuWO4•2H2O [7].

Conclusion
CuWO4 particles were prepared by polyol-hydrothermal process. The samples annealed by conventional method showed highly crystalline CuWO4 with CuO at 800 °C. While the HMA method showed reduce crystallite size. It is concluded from the comparison study, the HMA is useful for synthesising impurity free CuWO4 nanoparticles.
References
- Zheng JY, Song G, Kim CW, Kang YS (2012) Facile preparation of p-CuO and p-CuO/n-CuWO4 junction thin films and their photoelectrochemical properties. Electro chimica Acta 69: 340-344.
- GonzalezCCM, Du X, Dunford JL, Post ML (2012) Copper tung state thin- films for nitric oxide sensing. Sens Act B 173: 169-176.
- Chang Y, Braun A, Deangelis A, Kaneshiro J, Gaillard N (2011) Effect of thermal treatment on the crystallographic, surface energetics and photoelectrochemical properties of reactivity cosputtered copper tungstate for water splitting. J Phys Chem C 115(51): 25490-25495.
- Montini T, Gombac V, Hameed A, Felisari L, Adami G, et al. (2010) Synthesis, characterization and photocatalytic performance of transition metal tungstates. Chem Phys Lett 498(1): 113-119.
- Kumar RD, Karuppuchamy S (2014) Synthesis and characterization of nanostructured copper tungstate (CuWO4) for supercapacitors applications. Ceram Inter 40: 12397-12402.
- /a> ChenH, Xu Y (2015) Photocatalytic organic degradation over W-rich and Cu-rich CuWO4 under UV and visible light. RSC Adv 5: 8108-8113.
- Clark GM, Doyle WP (1966) Infra-red spectra of anhydrous molybdates and tungstates. SpectrochimicaActa 22(8): 1441-1447.






























