Flame Spray Pyrolysis Processing to Produce Metastable Phases of Metal Oxides
Gagan Jodhani1* and Pelagia-Irene Gouma1,2
1Department of Predictive Performance Methodologies, University of Texas at Arlington Research Institute (UTARI), USA 2Department of Materials Science & Engineering, University of Texas at Arlington, USA
Submission: March 30, 2017;Published: May 03, 2017
*Corresponding author: Gagan Jodhani, Department of Predictive Performance Methodologies, University of Texas at Arlington Research Institute (UTARI), USA, Email: gagan.jodhani@uta.edu
How to cite this article:Gagan J, Pelagia-Irene G. Flame Spray Pyrolysis Processing to Produce Metastable Phases of Metal Oxides. JOJ Material Sci. 2017; 1(2): 555557. DOI:10.19080/JOJMS.2017.01.555557
Abstract
Functional metal oxides can exist as different polymorphs. Meta stable phases of these oxides may be produced by flame spray pyrolysis (FSP). This is a scalable, rapid solidification process that may be used for nano powder synthesis. This review describes the key features of the FSP process and equipment. It also provides examples of the use of FSP process to obtain a meta stable oxide phases at RT which have unique properties.
Keywords:Flame spray pyrolysis; Ceramics; Polymorphism; Metastability
Introduction
When bulk metal oxides are subject to differing conditions of pressure and temperature, they crystallize in Metastable forms that are structurally different [1]. However, the same Metastable components can be formed in nanoscale at normal conditions of temperature and pressure. The formation of metastable compounds has been related to Gibb’s free energy; a material, during nucleation, will exhibit a polymorph that has lowest free energy at a particular temperature and pressure. At a given thermodynamic condition, both Metastable and stable phases may coexist, but only one of the two phases is stable, with minimal free energy, and the other must be metastable and may transform into a stable state [1]. The free energy of a polymorph is governed by size, temperature and pressure. A slight change in either one of the condition may affect the formation of a polymorph.
To achieve a metastable form at room temperature, rapid solidification/quenching of these materials [2,3]. A number of studies focused on the formation of metastable complexes at room temperature by obtaining nanocrystalline solids by quenching from high temperatures. Among the various methods used, flame spray pyrolysis is of prime interest as the process offers high temperature gradient and low residence time (start of nucleation-quenching) [4]. Thus, generating favorable conditions towards formation of metastable phases. The process and its uses are described below.
The FSP process
The earliest definition of the flame spray pyrolysis process has been: “pyrolyzing a suitable vapor of precursors that when decomposed give oxides with interesting catalytic properties”[5]. Flame spray pyrolysis is described as a break down process and it involves delivering a droplet of the precursor into the reactor by using an atomizer/ nebulizer. Since the reactor is at high temperature, the solvent evaporates and the remaining solute reacts with the surrounding gases. The composition of the final product is determined by the stoichiometric ratio of the solute dissolved in the precursor. The size and velocity of the droplets as well as the concentration of the precursor determine the particle size distribution of the powders deposited. Finally, the morphology of the nano particles is also affected by the surrounding gas flow rate, the temperature, and the type ofprecursor used [6,7] (Table 1).
Flame-spray pyrolysis, while enabling kinetic control of metastable polymorphs, is also a very effective and scalable industrial process to synthesize oxide nano particles in large quantities while maintaining an excellent product quality. It has been used for the dry, one-step synthesis of catalysts, sensors, biomaterials, phosphors, and even nutritional supplements, each of which would require high quality metastable materials to ensure the desired functionality and provides precedent for FSP synthesized material quality. Flame spray pyrolysis has high processing temperature (as high as 2800K) and the highest gas flow rate of most oxide processing techniques. Based on these two factors, flame spray pyrolysis is the only processing method that can provide very short residence times (~ms time scale) in the highest temperature gradient region and is thus a powerful synthesis method for production of metastable species through kinetic control [8]. Various flame configurations are used for the manufacturing of nano particles, such as premixed and diffusion flames run in co-flow, or counter-flow conditions. In the diffusion flame configuration, the fuel and the oxidizer diffuse into each other inducing flame reaction and particle formation, while in premixed flames the precursor and the combustible gases are mixed before they enter the reaction zone (flame).
Types of FSP
Flame spray pyrolysis has been categorized into three different types:
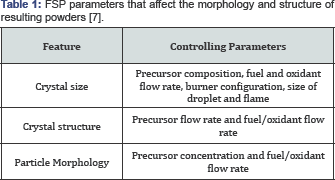
Vapor assisted flame spray pyrolysis (VAFS): A commonly used industrial process for the synthesis of ceramics, such as, fumed silica, alumina and pigmentary titania [9]. The process uses volatile metal precursors, which are evaporated and then fed into a flame. The metal particles are converted into oxides and particle formation occurs by nucleation from gas phase. The major drawback of the process is its need for volatile precursors, which limits the variety of products that can be generated through the process.
Flame aerosol spray pyrolysis (FASP): It uses noncombustible liquid metal precursors (such as aqueous solutions of metallic salts). The precursor liquid is dispersed into tiny droplets and pyrolyzed by an external flame [9]. The flame is usually produced by hydrocarbons or H2/O2. Upon evaporation, the particle formation process is similar to VAFS. Liquid feed
flame spray pyrolysis (LF-FSP):
This was developed by R.M Laine at University of Michigan, Ann Arbor [10]. The liquid fed FSP was able to overcome the drawback of FASP by fabricating more complex metal oxide particles. The process uses a metal oxide precursor in the form of liquid; its composition is generally in the form of metal chlorides, metal nitrates or metal acetates dissolved in aqueous or alcohol based solutions [11] (Figure 1). The process features short residence times (a few milliseconds) and results in formation of homogenous, nano sized particles [12].
Components of the FSP
Atomizer: The purpose of Atomizer is to create droplets. Three different kinds of atomizers [13] exist and are described below:
Ultrasonic nebulizer: Used to form sprays using highfrequency vibrations and converting liquid into mist. The mist enters the flame and follows the VAFS route, giving small particles.
Two-fluid nozzle: A device that disperses liquid into spray, the breakup of liquid into spray occurs with the help of an atomizing gas.
Electro-sprayer: It uses high voltage to convert liquid into aerosol. It is useful for producing ions from macromolecules.
A two-fluid nozzle is the most commonly used atomizer for FSP system.
Burner: The purpose of burner is to generate a flame. Two different types of burner exist, which are a premixed burner and a diffusion burner. The premixed burner mixes the fuel and oxidant [13]. FSP generates a self-sustaining flame so that the burner system for it is an ignition source. The advantage for it is that it could directly control the particle size by controlling the combustion enthalpy and the metal concentration of the precursor [4].
Collector: The purpose of collector is to collect the final product from FSP process. Usually a substrate or a filter is used for collection of particles.
Liquid precursor selection
The selection of liquid precursor is an important step in FSP particle synthesis. The selection of metallic precursor and solvent should be performed with keeping various factors in mind, such as, melting/decomposition temperatures, miscibility and chemical stability. These factors govern the overall particle formation in flame. Nitrates, acetates and acetyl acetonates are the natural choices for FSP as they were economical and readily available. However, they do not always yield homogeneous morphology [4].
The low combustion enthalpies of precursors coupled with high melting points results in formation of in-homogenous particles [14]. However, this drawback can sometimes be remediated by processing parameters. Following is how the particle formation is affected by liquid precursor formulation.
Hollow particles are formed when the boiling point of solvent is less than the melting point of solute or if the metal precursor precipitates on the surface of solvent. These parameters can be overcome by providing sufficient heat to the precursor. For formation of homogenous particles the melting point of precursor solute should be lower than the boiling point of solvent.
Mechanism for particle formation in FSP
One droplet to one particle (ODOP): As the name suggests, the formation of particles through this mechanism is by conversion of a single droplet into a single particle. The first step involves solvent evaporation and allows solute to disperse in the reactor. The solute then reacts with carrier gas or one-another to form particles. In final step, the particles undergo sintering and densification. The resultant of ODOP mechanism, yields particles in the micron size range. A process usually follows ODOP when the melting point of solute is higher than solvent [4].
The particle size for ODOP [13] can be predicted and is given by:
dp=((C〖Md〗^3)/nρ)^(1⁄3)
Where,
Dp is the average particle size
C is the concentration of the droplet
D is the diameter of the droplet
M is the molecular weight of particles
N is the stoichiometric ratio
ρ is the particle density
One droplet to multiple particles (ODMP): The steps involved in this mechanism are similar to that of ODOP. The difference between the two mechanisms is based on the precursor. Precursors tend to follow ODMP if there is presence of an organic additive. The additive leads to crack down of particles during the sintering process, which results in formation of multiple particles. The particles obtained exhibit a wide size range and are usually nano-crystalline [13].
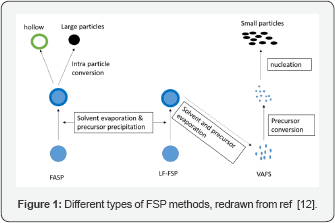
Gas phase mechanism: For this mechanism, the precursor is an organo metallic compound. The precursor evaporates quickly once it enters the flame, producing metal vapors. The formations of particles occur in various steps [4] and are given as a flow chart in (Figure 2) below: In the gas phase mechanism, the metal vapor may collide with the carrier gas to nucleate thereby forming clusters. Alternatively, the vapor may also nucleate on the surface of formed clusters resulting in new particles growth. The higher the number of clusters is being formed, the higher is the probability for them to collide with each other and form strong adhesive (physical) bonds or chemical bonds. The formations of bonds result in coagulation to produce nano particles. Finally, these nano particles produce single particles during coalescence process. The coalescence process results in aggregation of particles in the high temperature zone. To control the particle size by not allowing them to aggregate into larger particles, the lower residence time of precursor is needed [13]. The lower residence time can be achieved by a higher flow-rate of the precursor solution or high organic content of the organic precursor solvent, which results in bigger flame height.
Metastable phase formation
Flame spray pyrolysis has resulted in the formation of many metastable phases for ceramic oxides. The high temperature of operation coupled with rapid solidification and small particle sizes offer perfect conditions for formation of metastable polymorphs [4]. The size of particles formed through FSP are affected by various parameters such as composition of precursor, fuel and dispersion/oxidant flow rate, size of droplet and temperature of flame [15]. These parameters are interrelated as a high oxidant flow gives rise to low flame temperature. A non-combustible solute lowers the flame temperature, and lower oxidant flow rate decreases the droplet atomization and yields larger droplet. An optimum particle size and phase distribution, as well as tailored polymorph selection can be obtained from the same precursor by properly adjusting the processing parameters. For example, a metastable tetragonal- BaTiO3 was produced using FSP [16], from organic precursors of Ba and Ti dissolved in HNO3 and citric acid. Pure phases with low particle sizes were achieved for the precursor with the highest concentration of citric acid. This finding was associated with lower residence time of the vapors. In another study by a different group, the same recipe was used except citric acid was replaced by water [17]. The addition of water to the precursor mixture resulted in lower flame temperatures, bigger particle sizes, and the formation of a cubic-BaTiO3 phase. The lower flame temperature due to the addition of water increased the residence time for the particles which in turn led to formation oflarger particles and stabilization of the cubic polymorph. Organic precursors (organic solute in organic solvent) are the best choice forthe formation of metastable polymorphs as they offer a high temperature gradient and low residence time.
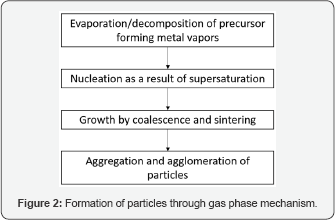
Tungsten trioxide is a well-known metal oxide that finds widespread use in gas sensing, electro chromic, and catalytic applications [18-20]. Polymorphic transformations of the pseudo-cubic lattice result in a wide variation in its electronic properties. WO3 usually crystallizes in one of the following lattice configurations- triclinic, monoclinic, orthorhombic, and tetragonal (Figure 3). All of these polymorphs are distorted forms of a cubic ReO3 lattice, with increasing order of crystallographic symmetry from the triclinic to tetragonal lattice. The crystal lattice is composed of a framework of metal-oxygen octahedral units, where the metal atoms are located at the center of the oxygen octahedron with varying amounts of metal-oxygen bond lengths and thus varying amounts of octahedral distortion. This distortion in turn serves to stabilize the different polymorphs. (Figure 4) demonstrates that high quality ε-WO3 nano powders have been synthesized using FSP by our group. The ε-phase for WO3 is very important for gas sensing applications as it acts as a selective sensor for acetone detection [19].
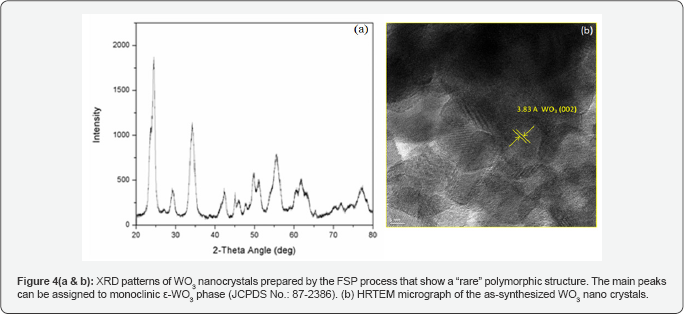
Conclusion
The operating principle and key features of the flame spray pyrolysis process have been reviewed in this work. It has been shown that this atomization process may be fine-tuned to guide the selection of particular polymorphs of metal oxides as the final product. Material precursor parameters, such as the solvent used, play a key role to determining the final particle size and the phase of the powders produced.
Acknowledgement
This work was made possible with funding from the NSF (DMR 1106168).
References
- Wang CX, Yang GW (2005) Thermodynamics of metastable phase nucleation at the nanoscale. Materials Science and Engineering: R: Reports 49(6): 157-202.
- Gilgien P, Zryd A, Kurz W (1995) Metastable phase diagrams and rapid solidification processing. ISIJ International 35: 566-573.
- Shu-qing Y (2014) Influence of Rapid Solidification on the Micro structural Development of Zinc-Aluminum Alloys. Metallography Microstructure and Analysis 3(2): 147-151.
- Teoh WY, Amal R, Mädler L (2010) Flame spray pyrolysis: An enabling technology for nanoparticles design and fabrication. Nanoscale 2(8): 1324-1347.
- Wold A, Gao YM, Miller D, Kershaw R, Dwight K (1996) Synthesis of Catalytic Materials by Spray Pyrolysis. In: Moser WR (Ed.), Advanced Catalysts and Nano structured Materials: Modern Synthetic Methods. Academic Press, USA, pp. 505-515.
- Okuyama K, Lenggoro WI (2003) Preparation of nano particles via spray route. Chemical Engineering Science 58(2003): 537-547.
- Strobel R, Pratsinis SE (2007) Flame aerosol synthesis of smart nano structured materials. Journal of Materials Chemistry 17: 4743-4756.
- Moser WR, Lennhoff JD, Cnossen JE, Fraska K, Schoonover JW (1996) The Preparation of Advanced Catalytic Materials by Aerosol Processes. In: Moser WR (Ed.), Advanced Catalysts and Nano structured Materials: Modern Synthetic Methods Academic Press, USA, pp. 535-562.
- Pratsinis SE (1998) Flame aerosol synthesis of ceramic powders. Progress in Energy and Combustion Science 24(3): 197-219.
- Author (2005).
- Helble J (1998) Combustion aerosol synthesis of Nanoscale ceramic powders. Journal of Aerosol Science pp. 721-736.
- Strobel R, Alfons A, Pratsinis SE (2006) Aerosol flame synthesis of catalysts. Advanced Powder Technology 17: 457-480.
- Purwanto A, Wang WN, Okuyama K (2011) Flame Spray Pyrolysis. In Handbook of Atomization and Sprays: Theory and Applications Ashgriz N (Ed.), Springer, Boston, USA, pp. 869-879.
- Jang YJ, Simer C, Ohm T (2006) Comparison of zinc oxide nano particles and its nano-crystalline particles on the photo catalytic degradation of methylene blue. Materials Research Bulletin 41: 67-77.
- Mädler L, Kammler HK, Mueller R, Pratsinis SE (2002) Controlled synthesis of nano structured particles by flame spray pyrolysis. Journal of Aerosol Science 33: 369-389.
- Jung DS, Hong SK, Cho JS, Kang YC (2008) Nano-sized barium titanate powders with tetragonal crystal structure prepared by flame spray pyrolysis. Journal of the European Ceramic Society 28: 109-115.
- Purwanto A, Wang WN, Lenggoro IW, Okuyama K (2007) Formation of BaTiO3 nano particles from an aqueous precursor by flame-assisted spray pyrolysis. Journal of the European Ceramic Society 27: 4489- 4497.
- Jodhani, G, Huang J, Gouma P (2016) Flame Spray Synthesis and Ammonia Sensing Properties of Pure α-MoO3 Nano sheets. Journal of Nanotechnology Volume 2016(2016): Article ID 7016926.
- Wang L, Teleki A, Pratsinis SE, Gouma PI (2008) Ferroelectric WO3 Nano particles for Acetone Selective Detection. Chemistry of Materials 20: 4794-4796.
- Lee J, Gouma PI (2014) Flame-Spray-Processed CuO-WO2 Nano powders as Photo catalysts. Journal of the American Ceramic Society 97: 3719-3720.






























