Prevalence and Prognostic Relevance of Modifying Anatomical Features Influencing the Measurement of Fractional Flow Reserve in Complex Situations in Daily Practice
Sebastian Barth1*, Sebastian Kerber1, Michael Zacher2 and Martina Hautmann1
1RHÖNKLINIKUM Campus Bad Neustadt/Saale, Department of Cardiology, Bad Neustadt/Saale, Germany
2RHÖNKLINIKUM Campus Bad Neustadt/Saale, Department of Medical Documentation, Bad Neustadt/Saale, Germany
Submission: December 13, 2022; Published: January 05, 2023
*Corresponding author: Sebastian Barth, Department of Cardiology RHÖNKLINIKUM Campus Bad Neustadt Von-Guttenberg-Str. 11D - 97616 Bad Neustadt a. d. Saale, Germany
How to cite this article: Sebastian B, Sebastian K, Michael Z, Martina H. Prevalence and Prognostic Relevance of Modifying Anatomical Features Influencing the Measurement of Fractional Flow Reserve in Complex Situations in Daily Practice. J Cardiol & Cardiovasc Ther. 2023; 18(1): 555980. DOI: 10.19080/JOCCT.2023.18.555980
Abstract
Fractional low reserve (FFR)-guided percutaneous coronary intervention (PCI) has been shown to reduce the need for PCI and to improve clinical outcome. Several anatomical features and hemodynamic parameters are well known to influence FFR determination. We evaluated the prevalence and prognostic impact of modifying anatomical features influencing the FFR- measurement on survival, myocardial infarction and the need of recurrent coronary revascularization.
Methods
For 232 patients undergoing cardiac catheterization with FFR-measurement 2014 in our institute, a match partner (without FFR-measurement) could be found by using the propensity score method. The factors influencing the FFR measurement were categorized and determined for each patient.
Results
The majority of patients (90.6% with and 90.5% without FFR) had at least one modifying factor. The number of implanted stents (FFR: n=114 vs. no FFR: n=181, p=0.006), stented vessels (FFR: n=79 vs. no FFR: n=112, p=0.015), and total stent length (FFR: 2134mm vs. no FFR: 3162mm, p=0.029) were higher in the group without FFR-measurement. During follow-up of 42.9 ± 10.5 months patients with FFR- measurement had less frequent myocardial infarctions (FFR: n=0 vs. no FFR: n=10, p=0.001) and Redo- PCI´s (FFR: n=21 vs. no FFR: n=47, p=0.007). There was no difference between the mortality rates of the two groups (HR 0.781, 95% CI 0.463-1.318).
Conclusion
FFR-guided PCI is not associated with increased long-term mortality and leads to a significantly lower incidence of myocardial infarction even in complex situations. FFR-guided PCI results in less re- interventions and reduces the number and length of implanted stents.
Keywords: Fractional Flow Reserve; Coronary Heart Disease; Modifying Factors; Long-Term Mortality
Abbreviations: FFR: Fractional Flow Reserve; PCI: Percutaneous Coronary Intervention
Introduction
Functional assessment of the coronary stenoses during invasive coronary angiography using intracoronary pressure guidewires has become an indispensable tool in daily practice. The impact of measuring the fractional flow reserve (FFR) in the decision-making of stenoses with a luminal diameter reduction of 50- 90% has been demonstrated in several studies [1-3]. FFR-guided percutaneous coronary intervention (PCI) has been shown to reduce the need for PCI and to improve clinical outcome [4]. Furthermore, this method has proven to be cost-effective [5]. These demonstrated beneficial effects have resulted in this method being highly recommended in the current guidelines of the European Society of Cardiology (ESC) 2018 [6]. Nevertheless, several hemodynamic factors, the size of the perfusion territory, the minimal lumen diameter and lesion length as well as microvascular dysfunction and diseases that led to an increased myocardial mass due to a diffuse scarring of the myocardium by fibrous transformation are well known to influence FFR determination. For this reason, patients with such the FFRmeasurement influencing factors were excluded from large, randomized trials like DEFER, FAME and FAME 2 [3,7,8]. Despite clear evidence, many interventional cardiologists continue to rely on the visual assessment of diameter stenosis severity alone, rather than performing FFR [9]. Potential reasons include the uncertainty about the interpretation of FFR measurements, particularly in complex situations [10]. This finding is also confirmed by the results of the prospective ALKK (Arbeitsgemeinschaft Leitende Kardiologische Krankenhausärzte) coronary angiography and PCI registry of data of 38 hospitals from 2010 to 2013. Overall, FFR was performed in only 3.2% of coronary angiographies, whereas in real-world scenarios use has a wide range from 0.1 to 8.8% [11]. The aim of our analysis was to evaluate the prevalence of these modifying anatomical features influencing the FFR-measurement in patients who underwent cardiac catheterization in our institute and whether these factors negatively affect the well known benefits of FFR measurement on survival, myocardial infarction or the need of recurrent percutaneous coronary interventions (PCI) and coronary artery bypass grafting (CABG).
Materials and Methods
Patient selection
Out of 233 patients undergoing cardiac catheterization with FFR-measurement 2014 in our institute, a match partner (cardiac catheterization without FFR-measurement in 2014) could be found for 232/3.462 patients by using the propensity score method. The 232 patients with FFR measurement represent the study population, and the matched patients (without FFR measurement) the control collective.
Indication for coronary catheterization
The indication for coronary catheterization included acute coronary syndrome, persistent angina despite medication, dyspnea at rest or exertion or markedly positive stress test. In the rare case of acute coronary syndrome (ACS), FFR measurement was conducted in a non-culprit lesion vessel.
FFR measurements
The FFR is defined as the ratio between distal coronary pressure and aortic pressure, both measured simultaneously at maximal hyperemia. Distal coronary pressure was measured with a coronary pressure guidewire (Volcano Verrata Pressure Wire®,Volcano, San Diego, CA 92130 USA). Maximal hyperemia was induced by intravenous adenosine, administered at 140 g/ kg/min via a central vein. Hyperemic pullback recordings were performed in case of diffuse diseased arteries to discriminate focal from diffuse disease. In the FFR group, an FFR ≤0.75 of a particular stenosis was considered as functionally significant and was revascularized with either a stent or a bypass. Percutaneous coronary intervention (PCI) was performed with drug eluting stents (DES) and/or bare metal stents (BMS).
Data acquisition
Datasets of 2014 of our patients were retrieved from the ALKK coronary angiography and PCI registry, including information about medical history, indication for the procedure, the procedure itself and complications until hospital discharge. All data, obtained using the standardized questionnaires of the ALKK registry, were analyzed centrally at the Karl Ludwig Neuhaus Datenzentrum in Ludwigshafen, Germany. Additionally, further metrics (i.e. invasive measurements, perfusion territory, microvascular dysfunction, etc.) were retrieved from electronic patient records at the time of catheterization and re-evaluation of the recorded cardiac catheterization at our institute. All variables were documented in a categorical fashion.
Obstructive diameter stenosis was defined as the presence of coronary stenosis equal to or greater than 50%. A patient was considered to be hypertensive if he or she had received such a diagnosis on the basis of persistently elevated systemic blood pressure greater than 140/90 mmHg. A patient was considered to have diabetes in case of increased levels of plasma glucose >200mg/dl after two hours in oral glucose tolerance test, after fasting or elevated glycated hemoglobin (A1C). The cardiovascular risk factor cigarette smoking was divided into three categories: smoker (smoking during the last two month), former smoker (smoker who quit smoking at least one month ago) and non smoker. Patients who have stopped smoking more than 20 years ago were not considered to have smoking as a risk factors. Family history was defined as coronary heart disease or stroke in first- or second-degree relatives under the age of 65 (females) and 55 (males). All patients gave informed consent, and the ALKK registry has been approved by the ethics committee of the Landesärztekammer Rheinland-Pfalz in Mainz, Germany.
Categorization of influencing factors of FFRmeasurement
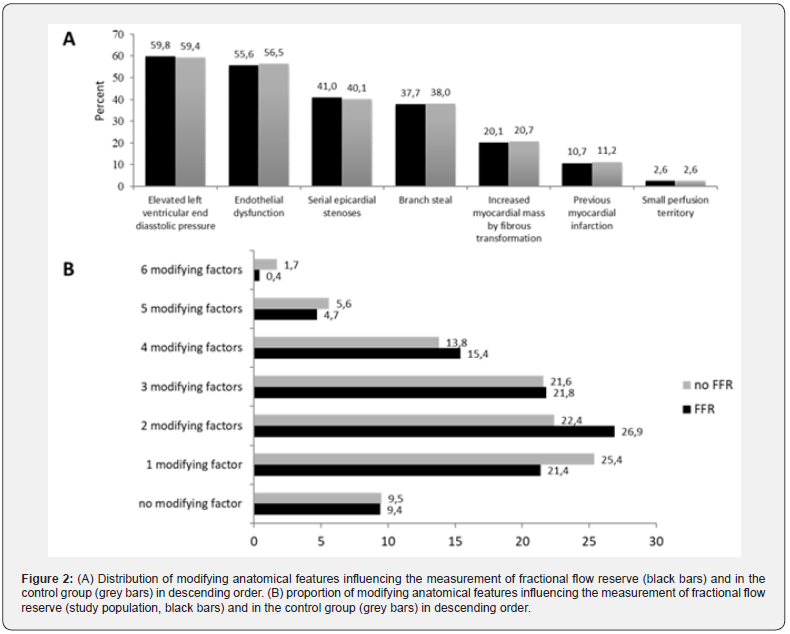
The factors influencing the FFR measurement were divided into seven groups: (1) elevated left ventricular end diastolic pressure, (2) endothelial dysfunction, (3) serial epicardial stenoses, (4) branch steal, (5) decreased myocardial mass by fibrous transformation, (6) previous myocardial infarction and (7) small perfusion territory. An elevated left ventricular pressure (1) was defined as >12mmHg in invasive measurement. Under the heading endothelial dysfunction (2) of the coronary artery examined with FFR, a diffuse atherosclerotic disease, a slow flow-phenomenon (TIMI 2) and prior stents were subsumed. The category branch steal (4) included a multi vessel disease (three vessel disease), abundant collaterals and bypassed vessels. Diseases that led to an increased myocardial mass due to a diffuse scarring of the myocardium by fibrous transformation due to pressure or volume loading have been summarized under point 5. These included a dilated cardiomyopathy with pronounced structural dilatation (LVEDD >56mm, LVESD >35mm), a severe impaired left ventricular function (LVEF <30%) and a left ventricular hypertrophy of >12mm.
Statistical Analysis
Quantitative variables were expressed as mean ± standard deviation. Qualitative data (nominal or ordinal scale) were given as frequencies and rates. For comparison between subgroups, the classical chi-square- test or Kruskal-Wallis test were used. P-values of a two-tailed test of 0.05 or less were considered significant. Data analysis of the ALKK registry was performed using SAS statistical analysis software, version 9.3 (SAS Institute, Inc., Cary, North Carolina. U.S.A.), and of the Cardiovascular Center Bad Neustadt/Saale using IBM SPSS Statistics for Windows, Version 24.0. Armonk, NY: IBM Corp.
Propensity-score matching
We used nearest neighbor propensity-score matching to ensure that the two patient-groups, with and without FFR, were comparable in terms of patient characteristics. The propensityscore was estimated by the use of all listed variables in Figure 1 [12-14]. Based on the calculated score, every patient of the group with FFR was matched to the closest patient of the group without FFR. Furthermore, the propensity-score’s tolerable deviation of each match was defined in advance with a caliper (“maximum distance”), therefore consequently excluding bad matches with high differences. The caliper was set at 0.2 ∗ propensity-score’s standard deviation, which reduced the difference between the groups by >95% [12].
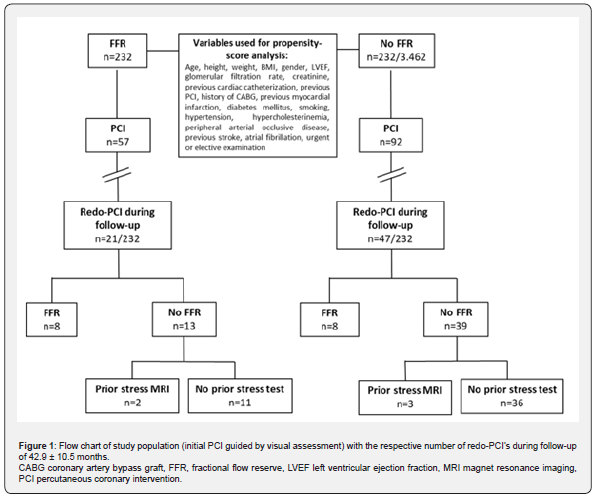
Results
Patient characteristics
Mean patient age was 68±10 years (FFR) and 67±11 years (no FFR). 67 out of 232 (28.9%) of the group with FFR-measurement and 63 out of 232 (27.2%) without FFR-measurement were female. The mean left ventricular ejection fraction (LVEF) was 56±13% (FFR) and 57±12% (no FFR). 18 out of 232 (7.8%) in the FFRgroup and 16 out of 232 (6.9%) without FFR-measurement had an impaired LVEF of ≤30%. The two groups were homogeneous with regard to previous cardiac catheterization (FFR: 65.1% vs. no FFR: 65.1%), PCI (FFR: 47.0% vs. no FFR: 49.1%), history of coronary artery bypass grafting (FFR: 7.8% vs. no FFR: 7.3%) and myocardial infarction (FFR: 26.7% vs. no FFR: 21.6%). Further patients characteristics are summarized in Table 1.
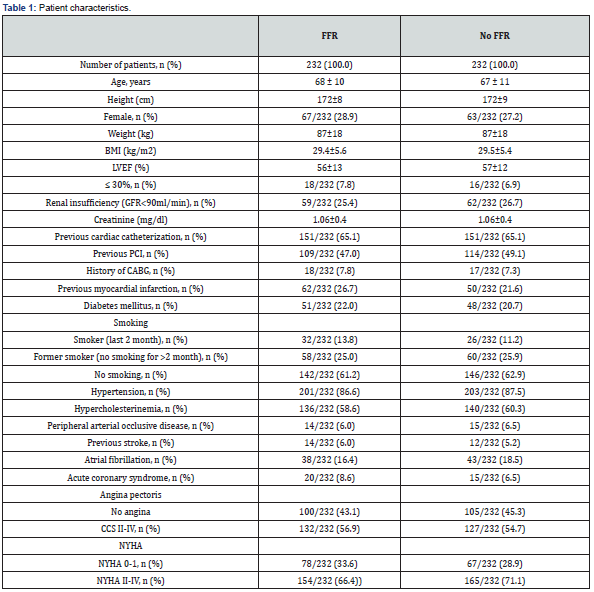
BMI body mass index, CAD coronary artery disease, CABG coronary artery bypass grafting, CCS Canadian Cardiovascular Society grading of angina pectoris, LVEF left ventricular ejection fraction, NYHA New York Heart Association, PCI percutaneous coronary intervention.
Intra- and post-procedural parameters
Both groups were homogenous for the prevalence of left main stenosis (FFR: 5.6% vs. no FFR: 3.5%, p=0.264). In the vast majority of procedures, drug-eluting stents were implanted in both groups (FFR: 83.3%vs. no FFR: 82.9%, p=0.497). The number of implanted stents (FFR: n=114 vs. no FFR: n=181, p=0.006), total stent length (FFR: 2134mm vs. no FFR: 3162mm, p=0.029) and the number of stented vessels (FFR: n=79 vs. no FFR: n=112, p=0.015) were higher in the group without FFR-measurement. During follow-up of 42.9 ± 10.5 months patients with FFRmeasurement had less frequent myocardial infarctions (FFR: n=0 vs. no FFR: n=10, p=0.001) and Redo-PCI´s (FFR: n=21 vs. no FFR: n=47, p=0.007). Further information is listed in Table 2.
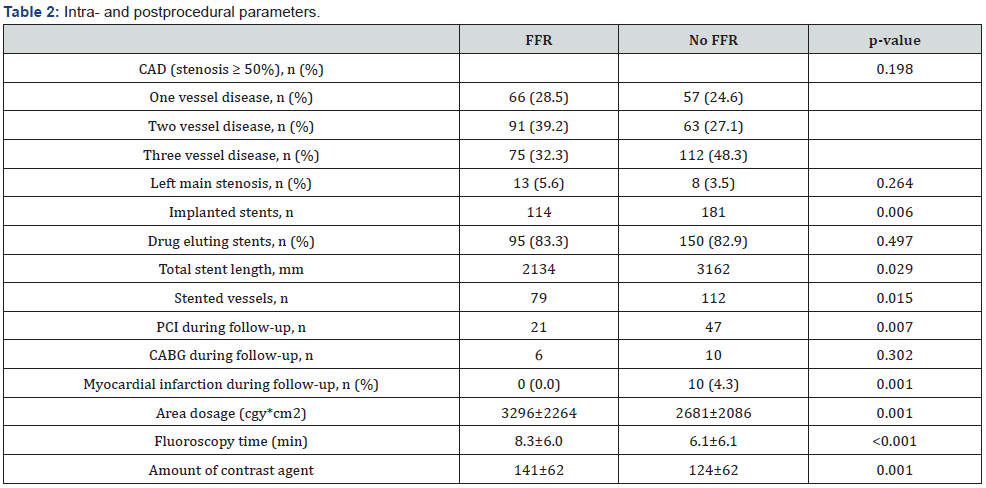
CAD coronary artery disease, PCI percutaneous coronary intervention.
Recommendations
The measurement of the FFR was associated less frequently with a recommendation of PCI (FFR: n=57, 24.4% vs. no FFR: n=92, 39.7%, p<0.001), while there was no difference regarding the recommendation of surgical treatment between both groups (with FFR 12.1% vs. without FFR 9.5%, p=0.387) (Table 3).
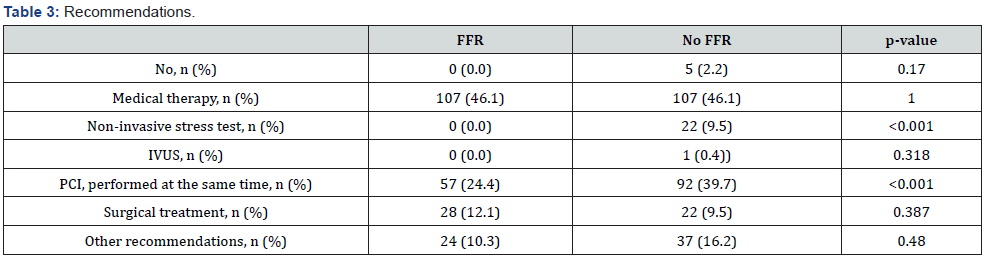
IVUS intravascular ultrasound, PCI percutaneous coronary intervention.
In-hospital complications
There were no differences in bleeding or puncture site complications (FFR: n=5, 2.2% vs. no FFR: n=5, 2.2%, p=1.000). Both groups had neither periprocedural complications rates resulting in death (up to 36 hours after the procedure), non fatal myocardial infarction nor stroke, MAC(C)E rates, rescue PCI or CABG.
Prevalence and distribution of confounding factors
59.8% of the patients, examined with an FFR-measurement, and 59.4% of the patients without FFR- measurement had an elevated left ventricular end diastolic pressure as the most common confounding factor. The prevalence of the other confounding factors is listed in descending order in Figure 2A. 9.4% of the patients with FFR-measurement and 9.5% of the patients without FFR-measurement had no confounding factor, while the vast majority (90.6% with FFR and 90.5% without FFR) had at least one factor influencing the FFR-measurement. Four or more factors were found in a fifth of the patients in both groups (Figure 2B).
Mortality rates
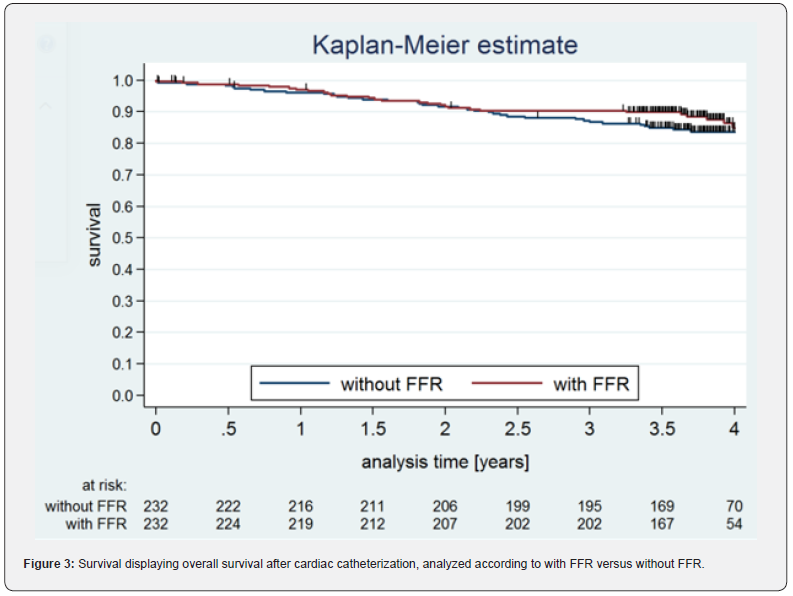
There was no significant difference between the mortality rates of the two groups (HR 0.781, 95% CI 0.463- 1.318). After one year 15 deaths in the group without FFR-measurement (survival rate 96.0 ± 0.01%) and 12 deaths in the group with FFR-measurement (survival rate 96.9 ± 0.01%) were reported. The four-year survival rate was 83.6 ± 0.03% (without FFR) and 84.8 ± 0.03% (with FFR). The Kaplan-Meier curves of all patients (n=464) divided into both subgroups are shown in Figure 3.
Discussion
According to the current European guidelines on myocardial revascularization, the assessment of a functional relevance of intermediate non-culprit stenoses represents the decisive gold standard for determining the appropriate treatment strategy [4]. The potential benefit of revascularization depends on the presence and extent of myocardial ischemia [7]. These recommendations are based mainly upon the results of the DEFER, the FAME and FAME-2 trial [3,7,8]. In these studies, however, there were numerous exclusion criteria. In the FAME study, the excluded patient groups comprised the following: patients with angiographically significant left main CAD, previous coronary artery bypass surgery, recent history of STEMI, impaired left ventricular function (<30%), left ventricular hypertrophy (>13mm), cardiogenic shock or extremely tortuous or calcified coronary arteries. In DEFER, patients with a total occlusion of the target artery, acute Q-wave infarction, or unstable angina documented by transient ST-segment abnormality as well as patients with small-sized target arteries (reference diameter <2.5mm) were excluded. Furthermore, the majority of the patients in the DEFER study had a single stenosis of uncertain functional severity in one single coronary artery. In daily practice, patients in the catheterization laboratory more often have multivessel disease, often with one or more angiographically severe stenoses and comitant intermediate stenoses [15,16]. As demonstrated, there are numerous factors that influence the FFR measurement.
Hyperaemia following maximum vasodilatation of the coronary microvasculature through adenosine administration is required for the assessment of a lesion-specific coronary ischemia. In presence of microvascular dysfunction due to an endothelial dysfunction, serial epicardial stenoses in a diffuse coronary artery disease, a branch steal effect, an increased myocardial mass by fibrous transformation as found in hypertrophic and/or dilated cardiomyopathy and previous myocardial infarction, the extent of achieved maximum hyperaemia will be less as compared to healthy individuals. Uncertainty about data acquisition and interpretation in these specific situations seem to be an explanation why the FFR measurement is not widely used in clinical practice [11].
In our study, we deliberately included all patients in whom an FFR measurement was performed in daily practice. The proportion of patients who would have been excluded in the FAME- and DEFER-study is more than half of our study population. In particular, left ventricular hypertrophy with a consecutive elevated left ventricular end diastolic pressure would have excluded more than half of all our patients. More than twothirds of our patients had at least two modifying anatomical features influencing the FFR-measurement. In addition to these large randomized trials, our investigation provides an important statement in the treatment of patients with numerous factors modifying the FFR-measurement in daily practice. Similar to the results of large, randomized studies with highly selected patient populations, FFR-guided PCI in our study population leads also to a significantly lower incidence of myocardial infarction and is not associated with increased long-term mortality. According to the large randomized trials DEFER, FAME and FAME 2, determination of the hemodynamic relevance of coronary artery stenoses with FFR results in less re-interventions and reduces the number and length of implanted stents even in complex situations. The comorbidities and anatomical features do not influence the result of the FFR measurement in the sense of false-negative or false-positive, but rather the functional significance of the angiographically evaluated epicardial stenosis, which is reflected in the FFR measurement.
Conclusion
FFR-guided PCI is not associated with increased longterm mortality and leads to a significantly lower event rate of myocardial infarction even in complex situations. FFR-guided PCI results in less re-interventions and reduces the number and length of implanted stents as well.
Limitations
Our observation is not a prospective, randomized trial. The selection of lesions measured with FFR was based on the operator´s visual interpretation of the angiogram together with clinical parameters. It is well- known that there is a high interobserver variability in assessing anatomic coronary stenosis severity. Therefore, the defined study population and their matchpartners are not representative of all patients undergoing invasive cardiac catheterization.
Acknowledgement
We are indebted to all participants of the registry and the staff of the Institute for Myocardial Infarction Research Foundation of Ludwigshafen, Germany.
References
- Tonino PA, Fearon WF, De Bruyne B, Oldroyd KG, Leesar MA, et al. (2010) Angiographic versus functional severity of coronary artery stenoses in the FAME study fractional flow reserve versus angiography in multivessel evaluation. J Am Coll Cardiol 55(25): 2816-2821.
- Van Belle E, Rioufol G, Pouillot C, Cuisset T, Bougrini K, et al. (2014) Outcome impact of coronary revascularization strategy reclassification with fractional flow reserve at time of diagnostic angiography: insights from a large French multicenter fractional flow reserve registry. Circulation 129(2): 173-185.
- De Bruyne B, Pijls NH, Kalesan B, Barbato E, Tonino PA, et al. (2012) Fractional flow reserve-guided PCI versus medical therapy in stable coronary disease. N Engl J Med 367(11): 991-1001.
- Pijls NH, van Schaardenburgh P, Manoharan G, Boersma E, Bech JW, et al. (2007) Percutaneous coronary intervention of functionally nonsignificant stenosis: 5-year follow-up of the DEFER Study. J Am Coll Cardiol 49(21): 2105-2111.
- Fearon WF, Bornschein B, Tonino PA, Gothe RM, Bruyne BD, et al. (2010) Economic evaluation of tractional flow reserve-guided percutaneous coronary intervention in patients with multivessel disease. Circulation 122(24): 2545-2550.
- Neumann FJ, Sousa UM, Ahlsson A, Alfonso F, Banning AP, et al. (2019) 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J 40(2): 87-165.
- Bech GJ, De Bruyne B, Pijls NH, de Muinck ED, Hoorntje JC, et al. (2001) Fractional flow reserve to determine the appropriateness of angioplasty in moderate coronary stenosis: a randomized trial. Circulation 103(24): 2928-2934.
- Tonino PA, De Bruyne B, Pijls NH, Siebert U, Ikeno F, et al. (2009) Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med 360(3): 213-224.
- Toth GG, Toth B, Johnson NP, De Vroey F, Di Serafino L, et al. (2014) Revascularization decisions in patients with stable angina and intermediate lesions: results of the international survey on interventional strategy. Circ Cardiovasc Interv 7(6): 751-759.
- Achenbach S, Rudolph T, Rieber J, Eggebrecht H, Richardt G, et al. (2017) Performing and Interpreting Fractional Flow Reserve Measurements in Clinical Practice: An Expert Consensus Document. Interv Cardiol 12(2): 97-109.
- Härle T, Zeymer U, Hochadel M, Zahn R, Kerber S, et al. (2017) Real-world use of fractional flow reserve in Germany: results of the prospective ALKK coronary angiography and PCI registry. Clin Res Cardiol 106(2): 140-150.
- Kuss O (2013) The z-difference can be used to measure covariate balance in matched propensity score analyses. J Clin Epidemiol 66(11): 1302-1307.
- Austin PC (2007) Propensity-score matching in the cardiovascular surgery literature from 2004 to 2006: A systematic review and suggestions for improvement. J Thorac Cardiovasc Surg 134(5): 1128-1135.
- Bertsekas DP, Tseng P (1998) Relaxation methods for minimum cost ordinary and generalized network flow problems. Operations Research 36(1): 93-114.
- Berger A, Botman KJ, MacCarthy PA, Wijns W, Bartunek J, et al. (2005) Long-term clinical outcome after fractional flow reserve-guided percutaneous coronary intervention in patients with multivessel disease. J Am Coll Cardiol 46(3): 438-42.
- Wongpraparut N, Yalamanchili V, Pasnoori V, Satran A, Chandra M, et al. (2005) Thirty- month outcome after fractional flow reserve-guided versus conventional multivessel percutaneous coronary intervention. Am J Cardiol 96(7): 877-884.






























