Heparin-Induced Venous and Arterial Thromboembolic Disease: Case Report and Literature Data
N Mahoungou-Mackonia1,2*, M El Mousaid2, N Brahim2, E Ovaga2, I Nouamou2, S Arous2, G Benouna2, A Drighil2, L Azzouzi2, C Couderq1 and R Habbal2
1Department of cardiology, Notre Dame de la miséricorde center hospital, Ajaccio, France
2Department of cardiology, chu-ibn ROCHD, Casablanca, Morocco
Submission: August 16, 2022; Published: November 30, 2022
*Corresponding author: Noël Maschell Mahoungou-Mackonia, Department of Cardiology, IBN ROCHD University Hospital, Morocco
How to cite this article: N Mahoungou-Mackonia, M El Mousaid, N Brahim, E Ovaga, I Nouamou, et al . Heparin-Induced Venous and Arterial Thromboembolic Disease: Case Report and Literature Data. J Cardiol & Cardiovasc Ther. 2022; 17(5): 555973. DOI: 10.19080/JOCCT.2022.17.555973
Abstract
HIT results from the development of F4P-specific IgG antibodies. It usually begins 5-14 days after initiation of heparin therapy with a drop in platelet count of more than 50% from a pretreatment value, associated in one in two patients with venous and/or arterial thrombotic complications. This is a rarer problem that occurs in 1-5% with UFH and 0.1-0.2% with LMWH and requires prompt management.
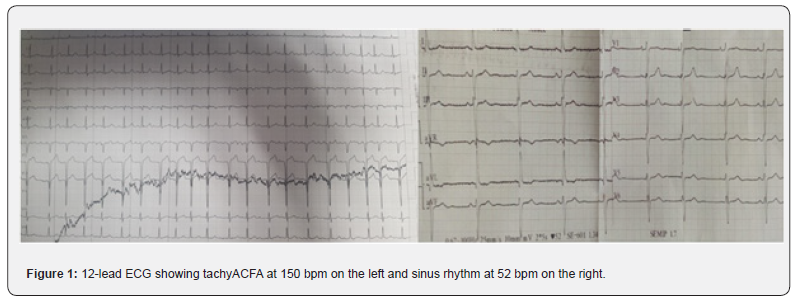
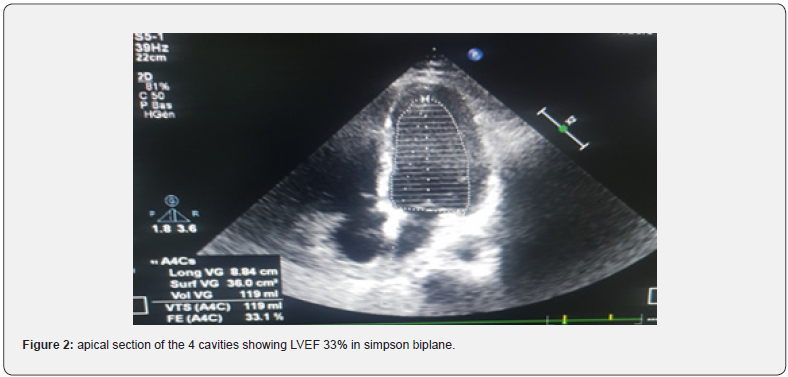
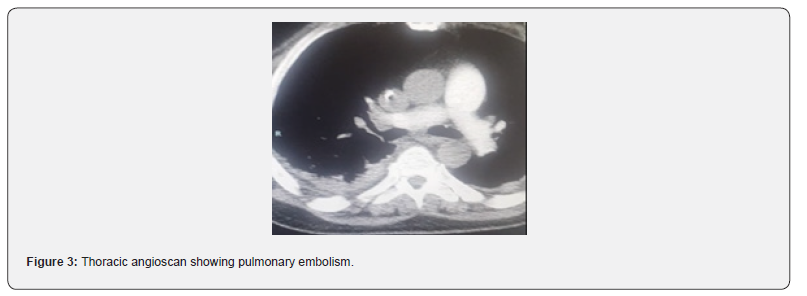
Forty-seven-year-old patient, alcoholic at 3g/d for 10 years, hospitalized for APO complicating an ethylic CMD, LVEF 33% in tachy ACFA at 150batt/min. The patient was treated with furosemide and risordan. Injectable Cordarone was administered and LMWH in curative dose for 7 days. The evolution was marked by a resumption of sinus rhythm, disappearance of crepitus rales, after one week, but an installation of thrombenia at 22000/mm3, a pulmonary embolism, left popliteal DVT and thrombosis of the iliac and then left femoral arteries. The diagnosis of HIT type 2 was confirmed by the presence of anti-PF4 antibodies in association with the platelet activation test. LMWH was stopped, the patient received Fondaparinux 7.5mg/d until platelets normalized after 10 days and then substituted with rivaroxaban for 6 months after clinical improvement and normalization of arteriovenous Doppler ultrasound and chest angioscan.
Keywords: Venous thromboembolic disease; Arterial thromboembolic disease; Heparin-induced thrombocytopenia
Abbreviations: HIT: Heparin-Induced Thrombocytopenia; F4P: Platelet factor 4; UFH: Unfractionated Heparins; LMWH: Low Molecular Weight Heparin; APO: Acute Pulmonary Oedema; CMD: Cardiomyopathy; ACFA: Complete Arrhythmia by Atrial Fibrillation; DVT: Deep Vein Thrombosis
Introduction
Heparin-induced thrombocytopenia (HIT) is a condition of acquired hypercoagulability of immune or non-immune origin, associated with disseminated cellular activation involving platelets, characterised by an abrupt decrease in their count, with a relative reduction of less than 20% or more than 50% of their initial value. It also involves monocytes and vascular endothelial cells and is associated with arterial and/or venous thrombotic events with increased morbidity in one out of two patients [1,2]. It usually begins 5-14 days after initiation of heparin therapy, with thrombotic events occurring at a rate of 5-10% per day during the first week, and reaching more than 50% cumulatively at one month [1,3]. HIT results from the development of specific IgG antibodies to a macromolecular heparin-platelet factor 4 (F4P) complex [1,3,4]. There are two types of HIT, type I with a frequency of 10-20%, characterised by a moderate decrease of less than 20% in platelet count. It is asymptomatic and corrects spontaneously and rapidly despite continued treatment. It is a benign and asymptomatic thrombocytopenia [5]. Type II is much rarer at 1-5% with unfractionated heparins (UFH) and 0.1-0.2% with low molecular weight heparin (LMWH). It is of immune origin with a delayed onset, but potentially severe thrombocytopenia and 50-60% thromboembolic complications [6]. Thus, we report a case of heparin-induced arterial and venous thromboembolic disease.
Case Report
47-year-old patient, 3g/d alcoholic for 10 years, hospitalised for Acute pulmonary Oedema complicating Ethyl Cardiomyopathy, LVEF 33% in tachy ACFA at 150 beats/min. The patient was treated with furosemide 250mg injection and risordan 1mg/ dr. The tachyACFA was controlled with Cordarone loading dose and then maintenance dose, low molecular weight heparin (LMWH) in curative dose was administered for the prevention of thromboembolic disorders for 7 days. The evolution was marked by a resumption of the sinus rhythm, disappearance of the crepitus rales after one week. The occurrence of thrombocytopenia in a progressive manner with a lower rate of 22000/mm3 was objectified in association with pulmonary embolism at high intermediate risk on thoracic angioscan. On venous and arterial Doppler ultrasound of the lower limbs, a deep venous thrombosis of the left popliteal vein and a thrombosis of the iliac and left femoral arteries were observed. The diagnosis of heparin-induced thrombocytopenia type 2 was confirmed by the presence of anti-PF4 antibodies in association with a positive platelet aggregation test. LMWH was stopped urgently, the patient received Fondaparinux 7.5mg/d until platelet count normalized to 198,000/mm3 in 10 days, then substituted with rivaroxaban 15mgX2/dr for 11 days then 20mg/dr for 6 months after normalization of venous and arterial Doppler ultrasound but also thoracic angioscanner (Figures 1-3).
Discussion
Heparin-induced thrombocytopenia (HIT) is a highly original model of drug-induced thrombocytopenia. The frequency of heparin-induced thrombocytopenia may vary according to the type of heparin administered without dependence on the route of administration, the underlying terrain, the indication for anticoagulant therapy, its duration and the dosages administered [2].
Heparins of bovine origin are more often incriminated than those of porcine origin. Although LMWH are implicated in the occurrence of HIT, their incidence is much lower than that of UFH, estimated at 0.1-0.5% [7].
The duration of heparin administration increases the incidence of HIT, whereas its dosage has little influence on its incidence, as even minute doses intended to maintain catheter patency are sufficient to generate it [1].
In terms of predilection, we have peripheral vascular disease, infection, orthopaedic or cardiovascular surgery requiring UFH administration, for which the risk of HIT is high at 5% for orthopaedics and 3% for cardiovascular surgery [4].
There are two types of HIT:
i. Type I, as described in the literature is non-immune, benign and asymptomatic correcting spontaneously and rapidly despite continued treatment. It is thought to be related to the direct interaction of platelets with the long polysaccharide chains of unfractionated heparin causing increased binding of fibrinogen and facilitating their elimination by the spleen [1].
ii. Type II is severe and typically occurs between the 5th and 15th day of heparin treatment, but may occur earlier if the patient has been exposed to a glycosaminoglycan (e.g. unfractionated heparin, low molecular weight heparin, pentosan polysulphate) and possibly “immunised” in the preceding weeks. According to Warkentin’s team.
HIT could also occur several days to several weeks after stopping heparin, but this possibility is probably very rare [8].
The pathophysiology is based on [1] the release of F4P and promotes the formation of heparin-F4P complexes. These large complexes are antigenic and induce antibody synthesis. These antibodies participate in the formation of immune complexes and lead to direct platelet activation through the interaction of the IgG Fc fragment with membrane FcγRII receptors (CD32). Other Ig (A or M) can directly activate other cells (lymphocytes, monocytes, neutrophils) but also indirectly activate platelets, e.g. after complement fixation. HIT is therefore associated with disseminated cellular activation involving platelets, monocytes and vascular endothelial cells, which can lead to full-blown coagulation. The combination of these cellular and plasma phenomena is responsible for the thrombosis and thrombocytopenia observed. More recently, it has been suggested that in rarer cases, type II HIT may be related to other mechanisms. For example, some patients develop IgA and/or IgM antibodies to F4P-heparin which appear to be as pathogenic as IgG. Others develop antibodies to different chemokines such as neutrophil-activating peptide (NAP- 2) and interleukin-8 (IL-8) [5]. The great heterogeneity of the antibodies generated and these “atypical” immunological profiles could partly explain the discrepancies between the clear clinical pictures of HIT and the biological tests.
The diagnosis of HIT cannot be based exclusively on clinical evidence, even if it is very suggestive [2]. Similarly, a positive sensitive biological test alone, outside a compatible clinical context, does not allow a diagnosis of HIT to be made. The diagnosis of HIT is therefore based on two types of argument:
the existence of at least one of the clinical signs associated with thrombocytopenia, namely
• venous thrombosis, pulmonary embolism, cerebral venous thrombosis
• Arterial thrombosis
• Skin lesions, skin necrosis, erythematous plaque
• Systemic reaction after bolus
• DIC;
Evidence of pathogenic heparin-dependent antibodies in the patient’s serum or plasma:
• Positive platelet activation test: platelet aggregation test, serotonin release test.
• Antigenic tests: H/FP4 ELISA, PVS/FP4 ELISA, Particle gel immunoassay
However, a positive antigen test alone does not confirm the diagnosis with certainty, it must be complemented by a platelet activation test [3].
In our study, this was a patient with a predilection for cardiomyopathy with cardiac arrhythmia who received LMWH for 7 days. In addition to severe thrombocytopenia, he eventually developed thromboembolic venous and arterial disease and the Elisa test showed anti-PF4 antibodies, supplemented by a positive platelet aggregation test. This confirms the diagnosis of HIT type II.
The treatment is based on the immediate cessation of heparin and any kind of heparin when HIT is suspected, without waiting for biological confirmation and the prescription of other anticoagulants, because this has created a real state of hypercoagulability. The attitude consisting [9] in rapidly switching to oral anticoagulant treatment alone should also be avoided, as not only does it not provide immediate protection but above all it may expose the patient to severe thrombotic accidents of skin necrosis or venous gangrene of the limbs. Thus (1) two therapies benefit from extensive experience and have, in France, a marketing authorisation (MA) for the treatment of HIT: danaparoid (Orgaran®) and recombinant hirudin (lepirudin [Refludan®]). Other alternative antithrombotic therapies are: Bivalirudin (Angiox®), Argatroban (Novastan®).
Fondaparinux (Arixtra®) is not recommended for the management of acute HIT. However, it can be proposed as an alternative treatment in case of a history of HIT in situations requiring preventive or curative non-heparinic anticoagulation [1]. In our case, due to the lack of first-line anticoagulant treatment and to see substitution, our patient received fondaparinux 7.5IU for 10 days until platelet count normalisation, then a relay was carried out with rivaroxaban 15mgx2/dr for 11 days and 20mg/d for 6 months.
Conclusion
HIT type II is a late onset autoimmune disease, usually severe due to accompanying thromboembolic events. Its diagnosis must be made early and based on a triad associating thrombocytopenia of at least 50% of the initial value, a clinical manifestation of the disease and biological confirmation. Management should be urgent after immediate discontinuation of heparin, based on the use of well-coded anticoagulants.
Disclaimer
The abstract has not been presented or published in any journal or conference.
Conflict of Interest:
None to declare.
Funding Disclosure: None to declare.
References
- Elalamy MM, Samama (2008) Heparin-induced thrombocytopenia: a complex paradox and multidisciplinary management.
- Pouplard C, Regina S, Gruel Y (2006) Heparin-induced thrombocytopenia: a severe clinico-pathological syndrome. Rev Francoph Lab 378: 49-58.
- Jevtic SD, Morris AM, Warkentin TE, Pai M (2021) Heparin-induced thrombocytopenia. CMAJ 193(20): E736.
- Gruel Y (2004) Heparin-induced thrombocytopenia and thrombosis: Pathophysiology, diagnosis and treatment. Rev Médecine Interne 25(1): 35-45.
- Greinacher A (2006) Heparin-induced thrombocytopenia: frequency and pathogenesis. Pathophysiol Haemost Thromb 35(1-2): 37-45.
- Plassat R, Cognet F, Ternisien C, Ménoret N, Dubus-Bausière V, et al. (2002) Heparin-induced thrombocytopenia: presentation of an observation with severe thrombotic complications and review of the literature. Ann Readapt Med Phys 45(5): 216-223.
- Davoren A, Aster RH (2006) Heparin-induced thrombocytopenia and thrombosis. Am J Hematol 81(1): 36-44.
- Warkentin TE, Kelton JG (2001) Delayed-Onset Heparin-Induced Thrombocytopenia and Thrombosis. Ann Intern Med 135(7): 502-506.
- Magnani HN, Gallus A (2006) Heparin-induced thrombocytopenia (HIT). A report of 1,478 clinical outcomes of patients treated with danaparoid (Orgaran) from 1982 to mid-2004. Thromb Haemost 95(6): 967-981.






























