Catheter Ablation of The Posterior-Septal Accessory Pathway in Patients with Wolf-Parkinson-White Syndrome Initially Present in Aberrant Atrial Fibrillation
Menon Tushar*, Pham William, Jain Shashank and Pham Richard
Department of Internal Medicine, Abrazo Community Health Network, Arizona, USA
Submission: August 16, 2022; Published: September 08, 2022
*Corresponding author: Tushar Menon, Department of Internal Medicine, Abrazo community Health Network, Arizona, USA
How to cite this article: GMenon T, Pham W, Jain S, Pham R. Catheter Ablation of The Posterior-Septal Accessory Pathway in Patients with Wolf-Parkinson-White Syndrome Initially Present in Aberrant Atrial Fibrillation. J Cardiol & Cardiovasc Ther. 2022; 17(4): 555972. DOI: 10.19080/JOCCT.2022.17.555972
Abstract
A 50-year-old man with no prior medical history went to the emergency department complaining of persistent palpitations, pressure in his chest, and shortness of breath with an EKG that revealed atrial fibrillation with aberrancy. Patient became hemodynamically unstable and was cardiovert. Post cardioversion EKG showed diffuse ST depressions and he was sent to the cath lab, The patient’s post-catheterization ekg displayed a short PR interval and a delta wave compatible with Wolff Parkinson White syndrome. Patient was treated with postero-septal catheter ablation. After his symptoms subsided, the patient was sent home.
Abbreviations: WPW: Wolff-Parkinson-White; ECG: Electrocardiographic; AF: Atrial Fibrillation; AP: Accessory Pathway; AVRT: Atrioventricular Reentrant Tachycardia; RF: Radiofrequency; SVT: Supraventricular Tachycardia; AV: Atrioventricular; SCD: Sudden Cardiac Death; Aps: Accessory Pathways
Introduction
The Wolff-Parkinson-White (WPW) syndrome is a preexcitation disease of the heart that results from aberrant cardiac electrical conduction through an auxiliary channel and may cause symptomatic and lethal arrhythmias. A short PR interval, extended QRS, and an early slurring upstroke (“delta” wave) in the presence of sinus rhythm are the hallmark electrocardiographic (ECG) findings of the WPW pattern or preexcitation. When a tachyarrhythmia and clinical tachycardia symptoms such palpitations, episodic dizziness, presyncope, syncope, or even cardiac arrest combine with an ECG pattern that is compatible with the observations mentioned above, it is referred to as the WPW syndrome [1]. The Wolff-Parkinson-White (WPW) syndrome raises the potential problems of atrial fibrillation (AF), a common arrhythmia, to a new level. In atrioventricular reentrant tachycardia (AVRT) and AF, Accessory pathway (AP) ablation is very efficient in eliminating errant conduction that occurs via the accessory pathway. The AP ablation is a curative procedure for the majority of WPW patients, however others re-main have prolonged AF episodes even post ablation Century (2011). Determining the potential causes for AF development in the WPW condition is thus crucial. There are a number of potential mechanisms that could be at play in the development of AF in the WPW syndrome, including intrinsic atrial muscle vulnerability, the electrophysiological characteristics of the AP, the effects of AP on atrial architecture, and spontaneous degeneration of atrioventricular reentrant tachycardia into AF.
Wolff-Parkinson-White (WPW)-associated arrhythmias are treated one of two ways the either by radiofrequency (RF) ablation of the accessory pathway (AP) or by antiarrhythmic medications. Catheter ablation is a way to treat irregular heartbeats, or arrhythmias, like atrial fibrillation (AFib), atrial flutter, or supraventricular tachycardia (SVT). Catheter ablation involves inserting a catheter through the femoral vein to access the heart and burn abnormal foci of electrical activity by direct contact or by isolating them from the rest of the atrium. It blocks the extra electrical activity and thus cures it [2]. Anti-arrythmics can used to slow AP conduction in specific circumstances such as in atriofascicular pathway-mediated supraventricular tachycardia. Typically, atrioventricular (AV) nodal-conduction blocking medications are avoided in the acute setting of WPW as fewer action potentials will pass through the AV node and more with pass through the accessory pathway worsening the underlying arrythmia.
Case presentation
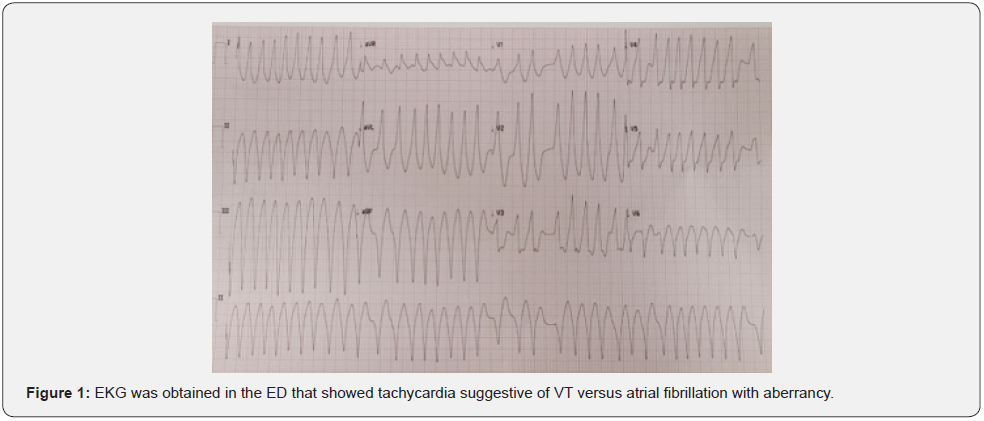
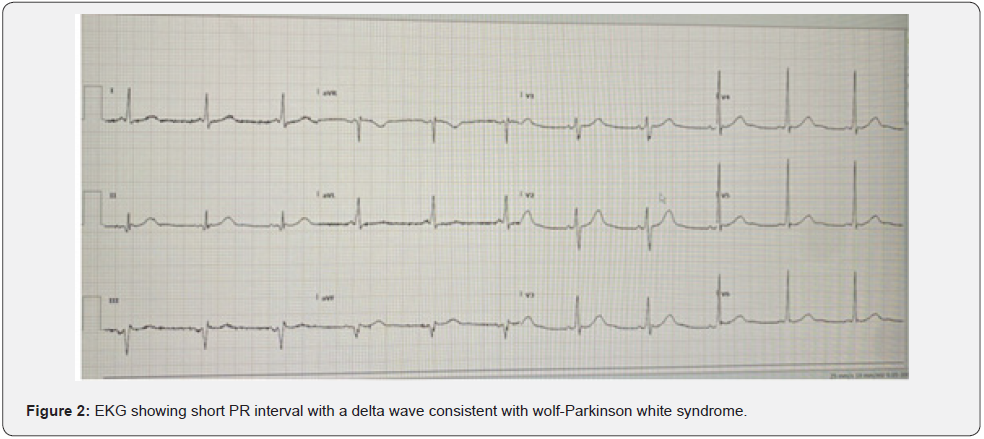
This is a 50-year-old male with no past medical history who presented to the ED for recurrent palpita-tions and chest tightness and shortness of breath. Patient had stated that he has been working long 14 hours plus days and because of that was drinking 2-3 red bulls every day for a month. EKG was obtained in the ED that showed tachycardia suggestive of VT versus atrial fibrillation with aberrancy (later determined to be atrial fibrillation with aberrancy- see (Figure 1). Initially his blood pressure was stable at 171/116. The patient was awake and alert, therefore decision was made to give a bolus of amiodarone. Shortly after, blood pressure significantly dropped down to systolic of 80s therefore decision was made to perform emergent cardioversion. He was given 10 mg of etomidate and cardioverted with 200 J with spontaneous return of sinus rhythm. EKG was obtained post cardioversion and showed diffuse ST depression in inferior and lateral leads as well as elevation in aVR. He was urgently taken to the Cath Lab where he was found to have slow flow phenomenon in the proximal LAD without evidence of plaque rupture, thrombus, or obstructive disease. Patient’s post Cath ECHO show normal ef. Post Cath there was short PR noted on the telemetry, on evaluation later that evening was found to to have a short PR interval with a delta wave consistent with wolf-Parkinson white syndrome (Figure 2) and therefore he was transferred to Abrazo Heart hospital and EP was consulted or radiofrequency ablation. Patient underwent successful SVT ablation of the extra assessor postero-septal pathway ablated endocardial from the LA and epicardially via the CS. Patient’s symptoms resolved and he was discharged home.
Discussion
Wolff-Parkinson-White (WPW) syndrome has an incidence of 0.1 to 3/1000 in healthy people and a prevalence of 0.1 to 0.3 percent in the general population. The incidence is greater in men and gradually decreases with age. Most people with an additional route are asymptomatic for the duration of their lives, and the risk of sudden cardiac death (SCD) is only 0.6 percent [2]. Those who have symptomatic arrhythmias and a WPW pattern on an ECG are said to have the WPW syndrome [3]. Preexcitation is shown on the ECG as a result of the atrium and ventricle having an aberrant connection through an auxiliary channel. Several kinds of accessory routes exist such as: The atrial and ventricular myocardium being directly connected via the bundle of Kent or Mahaim fibers which connect the atrium or AV node to the ventricular fascicles (atriofascicular or nodofascicular, respectively), which traditionally exhibit decremental conduction characteristics and only conduct in the antegrade direction. According to reports, 0.1-0.3% 1 of the general population has extra accessory pathways [4]. A frequent arrhythmia with several potential side effects, atrial fibrillation (AF) takes on a new dimension in the setting of the Wolff-Parkinson-White (WPW) syndrome. Although AP ablation is a curative treatment for the majority of WPW patients, some individuals continue to have prolonged AF episodes even after the AP has been removed. Although atrioventricular reentrat tachycardia (AVRT) and AF are almost always eliminited by AP ablation, it does not always prevent recurrences of AF in certain individuals [5]. Determining the potential causes for AF development in the WPW condition is thus crucial. It is crucial to recognize that AF, which may generate ventricular fibrillation due to a quick ventricular response, may be fatal in cases with WPW syndrome and fast anterograde AP conduction.
There are a number of potential mechanisms that could be at play in the development of AF in the WPW syndrome, including intrinsic atrial muscle vulnerability, the electrophysiological characteristics of the AP, the effects of AP on atrial architecture, and spontaneous degeneration of atrioventricular reentrant tachycardia into AF. AF has been linked to focal activity, numerous reentrant wavelets, and macroreentry, all of which may be further influenced by the autonomic nervous system. Ectopic beats coming from the pulmonary veins and other places may potentially start AF. Thanavaro & Thanavaro [6] Numerous studies showed a reduction in the incidence of AF after the effective removal of the AP, indicating that the AP may be crucial in the development of AF. Although some individuals with the WPW syndrome remain have AF even after a successful AP ablation, this suggests that there are likely other processes at play. In WPW the bunde of Kent allows for rapid anterograde conduction through the accessory pathway which can outperform slower atrioventricular (AV) node conduction. This route causes the ventricles to depolarize very quickly, which leads in distinctive ECG alterations such a short PR interval, broad QRS complex, and the almost pathognomonic delta wave. The auxiliary route may not conduct in an antegrade manner in “concealed” WPW syndrome, making it difficult to detect any electrocardiographic irregularities at baseline. The majority of WPW patients experience orthodromic AV reentrant tachycardia (orthodromic AVRT), which is characterized by an abnormally narrow QRS morphology and paroxysmal AV reentrant tachycardia (AVRT) caused by anterograde conduction through the AV node followed by retrograde conduction through the bundle of Kent. Chiang et al. [7] During AF, these individuals often do not exhibit quick preexcitatory responses, which is probably caused by a block or an antegrade conduction delay of the accessory route relative to the AV node. Contrarily, individuals with antidromic AVRT, in which anterograde conduction via the accessory channel is followed by retrograde conduction through the AV node, may have wide QRS tachycardia [8].
Additionally, individuals with pre-existing bundle branch blockages may experience this circuit. Pre-excited AF causes the atria to discharge more quickly than 300 impulses per minute, which masks delta waves, which are the primary electrocardiographic hallmark of WPW syndrome. Due to decremental conduction, an inherent repolarization trait that permits the node to conduct more slowly when it receives quicker signals, the AV node often blocks the majority of these impulses. However, 1:1 conduction is achievable with ventricular rates approaching 300 bpm when an auxiliary channel is used that does not have this built-in delay. Pre-Excited AF is thus classified as a malignant arrhythmia since it may develop into ventricular fibrillation and cause rapid heart death [9]. The posteroseptal accessory pathway in the Wolff- Parkinson-White syndrome is linked to a delta wave that is negative in the inferior electrocardiographic (ECG) leads and the occurrence of the earliest retrograde atrial activation close to the opening of the coronary sinus during arteriovenous insufficiency.
Posteroseptal accessory pathways (APs) are not true septal pathways but are located in the complex inferior pyramidal space involving the right atrium, right ventricle, left ventricle, left atrium, and coronary sinus and its branche.Targets for catheter ablation are similar to other APs including the earliest local ventricular activation during pre-excited rhythm and early atrial activation during orthodromic atrioventricular (AV) reentrant tachycardia [4]. Catheter ablation involves inserting a catheter through the femoral vein to access the heart and burn abnormal foci of electrical activity by direct contact or by isolating them from the rest of the atrium. Reduced BNP levels, a normalized QRS length, mechanical resynchronization, and enhanced LV function follow radiofrequency ablation.
Conclusion
Wolff Parkinson White Syndrome (WPW) is considered to be a preexcitation abnormality that involves the presence of abnormal electrically conductive circuits between the atria and ventricles.
In it, antegrade conduction occurs over an accessory pathway. If atrial fibrillation develops, this is a medical emergency as very rapid ventricular rates can develop because of high conduction through AV nodes. Electrocardiographic features include a short PR interval, a delta wave, a broadened QRS complex, and alterations in the ST-T wave pattern. The most common and complete treatment of. WPW syndrome is done with radiofrequency catheter ablation, which destroys the aberrant electrical channel by making scars and thus blocking these accessory pathways.
References
- Malani S, Sethi KK, Dhall A, Chadha DS, Garg S, et al. (2007) WPW and preexcitation syndromes. J Assoc Physicians India 55 Suppl: 10-5.
- Antunes E, Rasteiro R, Martins S, Oliveira M, Oliveira E, et al. (2019) Atrial Fibrillation in Wolff-Parkinson-White Syn-drome. JACC: Case Reports 18(9): 821-827.
- Chung KY, Walsh TJ, Massie E (1965) Wolff-Parkinson-White syndrome. American Heart Journal 69(1): 116-133.
- Timmermans C, Smeets JL, Rodriguez LM, Vrouchos G, van den Dool A, et al. (1995) Aborted sudden death in the Wolff-Parkinson-White syndrome. Am J Cardiol 76(7): 492-494.
- Pietersen AH, Andersen ED, Sandøe E (1992) Atrial fibrillation in the Wolff-Parkinson-White syndrome. The American Journal of Cardiology 70(5): A38-A43.
- Thanavaro JL, Thanavaro S (2010) Clinical presentation and treatment of atrial fibrillation in Wolff-Parkinson-White syndrome. Heart & Lung 39(2): 131-136.
- Chiang CE, Chen SA, Tai CT, Wu TJ, Lee SH, et al. (1996) Prediction of Successful Ablation Site of Concealed Posteroseptal Accessory Pathways by a Novel Algorithm Using Baseline Electrophysiological Parameters. Circulation 93(5): 982-991.
- Centurión OA, (2011) Atrial Fibrillation in the Wolff-Parkinson-White Syndrome. J Atr Fibrillation 4(1): 287.
- Hamada T, Hiraki T, Ikeda H, Kubara I, Yoshida T, et al. (2002) Mechanisms for Atrial Fibrillation in Patients with Wolff-Parkinson-White Syndrome. J Cardiovasc Electrophysiol 13(3): 223-229.






























