The Impact of Age-Related Sarcopenia on Survival and Mortality in Patients with Chronic Heart Failure
Konstantin D Gospodinov* and Snezhana T Tisheva
Department of Cardiology, High Medical University-Pleven, Bulgaria
Submission: October 13, 2021; Published: December 16, 2021
*Corresponding author: Konstantin D Gospodinov, Department of Cardiology, High Medical University, Pleven 5800, Bulgaria
How to cite this article: Konstantin D G, Snezhana T T. The Impact of Age-Related Sarcopenia on Survival and Mortality in Patients with Chronic Heart Failure. J Cardiol & Cardiovasc Ther. 2021; 17(3): 555965. DOI: 10.19080/JOCCT.2021.17.555965
Abstract
There is an increasing Heart failure (HF) population resulting from the higher number of myocardial infarction (MI) survivors, widespread presence of diabetes, hypertension, cardiac heart disease (CHD), obesity and other chronic conditions. Cardiac dysfunction is the main factor that lead to reduced physical activity in patients with HF. The alternations in skeletal musculature often are present in the background of HF and can also contribute to fatigue and dyspnea. Sarcopenia is reduction in muscle mass and force and is wright to say that it is one of the signs of “getting old”. The modern views on sarcopenia are that it is an outcome form number of medical, behavioral and ecological factors, which are common in older people. Together with sedentary life-style it can be the main reason for disability in late stages of human life. In our study we examined the significance of reduced skeletal muscle tissue on mortality and survival of patients with diagnosed HF.
Keywords: Chronic Heart Failure; Sarcopenia; Mortality
Introduction
There is an increasing HF population resulting from the higher number of MI survivors, widespread presence of diabetes, hypertension, CHD, obesity and other chronic conditions. Cardiac dysfunction is the main factor that lead to reduced physical activity in patients with HF. The alternations in skeletal musculature often are present in the background of HF and can also contribute to fatigue and dyspnea [1]. ”Cardiac skeletal myopathia” in older age combined with sarcopenia can lead to more severe functional impairment [2]. Two thirds of the cases with advanced HF have muscle fiber atrophia, reduced capillary density, bolder shape of the myofibers secondary to myofiber edema and accumulation of fibrose and adipose tissue. The alternations in muscle morphology and fiber orientation leads to further reduction in force generation capasicty.
The nature of muscle changes in sarcopenia is different. During aging there is a selective denervation and loss of fast motor units, fibers of type 2 are more prone to atrophy than type 1 fibers. There is 26% reduction in the cross section of fibers of 80 years old compared to those of 20 years old. After age of 80, both type of fibers are decreasing. Denervation and loss of fast motor units start from 60 years of age with around 3% tissue loss per year, which leads up to 60% loss of fibers at age of 80 or above. Adipose and fibrose infiltration are other important factor for muscle quality changes Figure 1 [3].
The selective advantage of bigger muscle mass in the late Paleolithic age can be main reason for increased death rate from sarcopenia, which is observed in many actual epidemiological studies. The discrepancy between our genome and relatively sedentary lifestyle can be important factor in inappropriately low gene expression of certain genes (for example gene a-actin) and to lead to progressive muscle mass reduction.
Drugs and nutrition have the potential to impact positively or negatively the progression of sarcopenia. For example NSAID, usually used by older people can lead to worsening of sarcopenia. The trials conducted among young healthy males show that NSAID are inclined to go through protein synthesis reaction, which are naturally present after eccentric exercise due to inhibition of the prostaglandin signals. It is yet unclear that same effects are present in older people with or without HF.
The effect of nutrition on skeletal muscle metabolism is not fully clear yet. The connection between protein intake and muscle mass is only a hypothesis. Some early reports don’t show significant effect of protein intake on the muscle mass and force in the elderly. Some newer data show that nutrition supplements in addition to physical exercise regime can prove useful for delaying sacrcopenia progression [4-6].
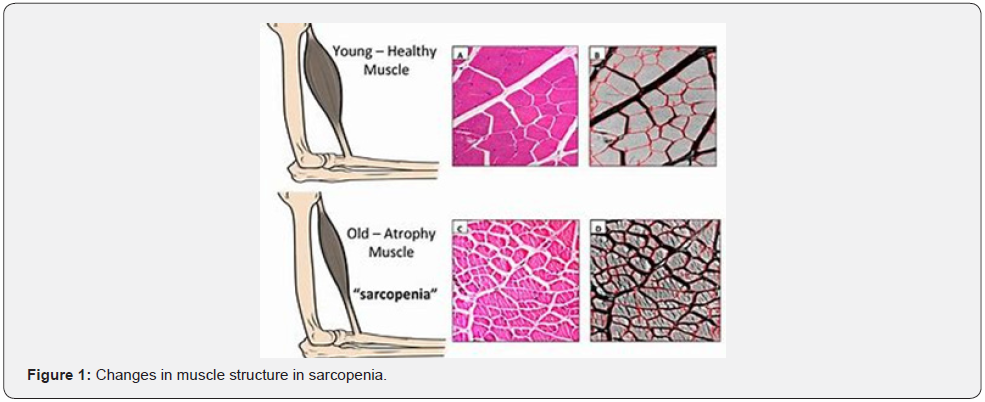
Patients with HF develop anorexia as result of nausea and secondary gastopathya due to intestine edema, which leads to malabsorbtion. Also, some of the drugs prescribed for HF, can lead to loss of appetite, such as ACE-I. and b-blockers. In addition, diuretics can promote electrolyte loss with urine. To summarize, the inadequate intake of basic elements and their loss makes HF prone to malnutrition and makes way for muscle depletion.
Other possible mechanism, which can explain sarcopenia, is the catabolic effect of chronic inflammation. The catabolic effects of anti-inflammatory cytokines like TFA-a, IL-1, IL-6 and of the muscle fibers are studied in invivo and invitro trials [7].
Epidemiological trials show that muscle force and in less, muscle mass, are connected with the levels of circulating IL-6 and TNF-a [7]. Ferrucci and colleagues show that the serum levels of IL-6, are a predictor for loss of muscle tissue force in elderly women. IL-6 also has negative role on muscle force [8]. In summary, these results show that inflammation is important reason of sarcopenia. In fact, IL-6 and IL-1 secretion is a main mechanism in the turnover of the muscle cell as answer to micro trauma after physical exercise. Inflammation markers usually are elevated in patients with HF. Inflammations also is part of the pathogenesis of sarcopenia. It is fundamental common factor between the two conditions. More specific, TNA-a and its soluble receptors promote muscle mass reduction and force during fiveyear follow-up among more than 2000 adults participating in the Health ABC study. Among the possible reasons for TNF-a elevation in HF is the endotoxin hypothesis. This suggests that intestinal edema, common in HF patients, will change permeability to endotoxin-like lipopolysaccharide (LPS), a powerful inflammatory stimulator and inductor of the monocyte activation. At last, even if TNF-a production is controlled mainly by mononucleal cells, the super expression is supported by catecholamines, and their concentrations are higher in HF patients, as an answer to myocardial damage and peripheral tissue hypoxia.
Shaap and coll. observe that higher levels of IL – 6 and CRP increase the risk of muscle force loss in a longitudinal trial about aging in Amserdam. IL-1, IL-6 and TNFa are linked with UPS and can induce anorexia and lipolysis, which leads to loss of body mass. If this thesis proves wright, it can open new therapeutic options. The newest data show that high levels of physical activity is linked with lower levels of circulating inflammation markers. This is in contrast with the fact that after vigorous physical activity, the serum levels of anti-inflammatory cytokines are extremely elevated [7,9]. It is proved thought that the peak levels of cytokines after exercise routine in people with systemic exercise regime, are becoming lower with time. Also in the same group, the overall levels of serum cytokines also are becoming lower at rest [10,11]. This is a possible mechanism for prophylactics for sarcopenia, especially in those with CHF. The latest trials focus on signal pathways, which are included in the genesis of muscle hypertrophy and atrophy. The first trial about signal pathways concerning muscle mass is conducted among patients with HIV and cancer. Because of super-expression of insulin-like growth factor – I (IGFI) in compensatory hypertrophy, its role is studied in many trials [10]. There is an idea, that IGFI can be useful in the treatment for sarcopenia, because it can directly stimulate the satellite cells and to activate the chain signal pathways which encourage cell proliferation. Age related reduction of growth hormone and the levels of IGFI are linked with reduced muscle mass and function.
Grelin is a peptide hormone, produced mainly in the belly area. It has pleiotropic effect including stimulation of growth hormone secretion. Lately grelin drew attention, because, as it seems, it inhibits the sympathetic nervous system activity thought vasodilatation in order regulation of appetite without growth hormone mechanisms, and also to inhibit anti-inflammatory cytokines [10]. Grelin also down-regulates secretion of leptin. (an (ob) gene product which reduces food intake and increases the energy expense for rest and regulates tissue growth factor beta one (TGF-b1)) [11].
Testosterone is studied as a possible factor in sarcopenia. Low levels of testosterone is common in patients with HF, and is believed that this helps for myocardial dis-function through peripheral vascular resistance alternation, increased cardiovascular load and cardiac output.
Angiotensin II is not only dependable for blood pressure control and heart muscle remodeling. It also play a role in muscle mass loss. In such model the loss of muscle tissue is mainly due to UPS mediated protein degradation. Other ambulatory trials show that implementation of ACE-inhibitors or ARBs is decreasing the level of myocyte apoptosis and generating of mitochondrial free radicals At the same time they improve NO synthesis and expression of rapamycin target (MOTR) in old rats [12].
Other option in sarcopenia control is the ubiquitinproteasome pathway, including two genes that are linked with skeletal muscle atrophy. These genes, MuRF1 and MAFbx, are super-regulated during the process of skeletal muscle atrophy and code ubiquitin ligases. These ligases connect and mediate ubiquitination of myofibril protein for upcoming degradation during muscle atrophy. Ubiquitin seems important for catabolic activity of many inflammatory cytokines which belong to the IL-6 superfamily [13]. Is UPS active in the sarcopenic muscle, that is still in discussion. The conducted trials, show different increase in UPS activity, in some significant, in others mild to none [14].
Myostatin, also know as grow factor 8, is a down-regulator of muscle mass. Myostatin is mainly expressed in skeletal muscle tissue as an inductor for muscle atrophy. Miostatin is also expressed in the myocardium, where it has anti-hypertrophic effect but also pro-fibrotic one. Myostatin – inhibiting and blocking agents should be carefully studied in pre-clinical models, before their safety and efficacy are determined in clinical trials in men [9].
In the last decade there is a hypothesis for accelerated elimination of nucleus cells duo to apoptosis, as a mechanism for sarcopenia. Several apoptosis pathways are linked age related muscle atrophy. In private, the TNF-a mediated pathway of apoptosis is active in the skeletal muscles of elderly rodents, suggesting its possible role in age related muscle loss [15]. Higher frequency of myocyte apoptosis is present in HF patients compared to healthy controls at the same age [15]. In the same trial cardiac cachexia is not linked with increase in myocyte apoptosis, but with increased fibrosis, suggesting different mechanism of muscle waste in HF patients and those with cachexia.
In normal physiological conditions, anaerobic metabolism generates small amount of reactive oxygen species (ROS) which are rapidly detoxified by antioxidant systems. When there is disbalance between pro-oxidants and antioxidants, oxidative stress is present. The production of ROS increases with age and it suggests that it is a factor in aging. ROS can accelerate muscle protein degradation as they are accumulate during contractile activity. Muscle enzyme systems (et. Catalase, glutation transferaza, superoxide dismutaza) are decreasing with age.
There are no evidence that ROS production and oxidative stress increase with aging, but there is convincing data, that tissue concentrations of serum antioxidant acceptors especially superoxydismutaza (SOD), are increasing with age.
Understanding the mechanisms, which lead to age-related and HF related sarcopenia, can be a key to developing new effective treatments that can improve patient’s quality of life.
The reduction of skeletal muscle mass has negative impact on metabolism, adaptation and immunological answer to the disease, reducing the ability of our organism to withstand the changes in the environment. Sarcopenia is a strong predictor for death rate independent from all other known risk factors and diseases.
In our cross-sectional observation, we analyzed some of the possible relationships between these two pathological processes.
Study Goal: The goal is to create a group of patients with HF II – IV class by NYHA, which were tested positive for sarcopenia through CT tissue densitometry and to compare the mortality and survival rate between HF non-sarcopenia patients and those with HF and sarcopenia.
Methods: We used documental and sociological, clinical, instrumental and statistical methods, to help us design, carry out and finish our study;
We created a group of patients, subsequently admitted to the clinic with HF, who fulfill all the including criteria (Age >/= 18y/o, present HF 2-4th grade by NYHA), doesn’t fulfill any of the excluding criteria (Age<18y/o, refusal of participating, oncological disease, connective tissue disease, any condition that will stop them from participating in the 6MWT) ,had signed the informed consent for undergoing the CT-test for the accession of muscle-tissue density.
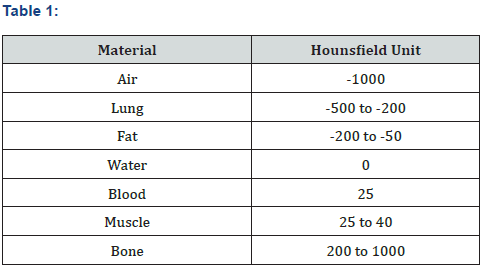
The CT test was made with a 16 slide GE machine. The area of examination was the quadriceps at 6 cm above the patella. The tissue density was made by and imagining diagnostic specialist, the evaluations was through the Hounsfield units scale. We considered values below 35 H.U. as “reduced muscle tissue density” (Figure 2) (Table 1).
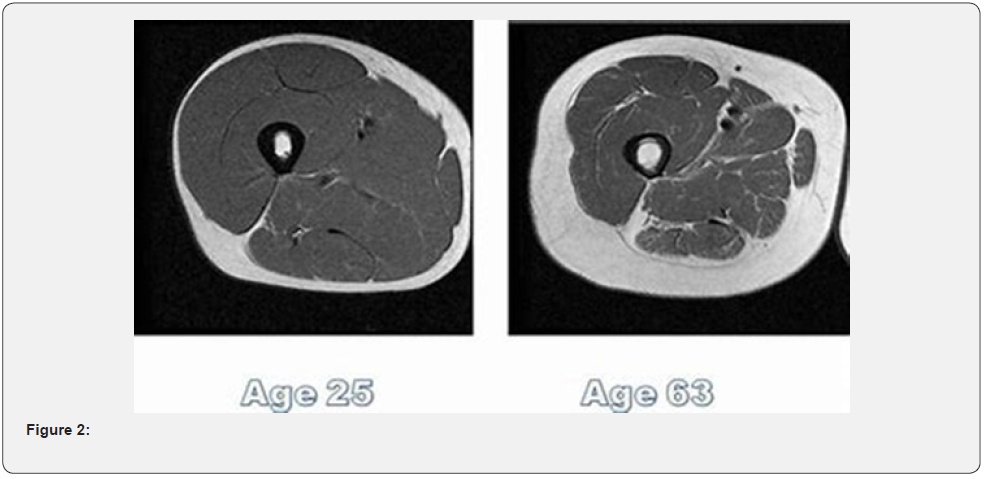
From the group, most of the patients were with NYHA Class III HF, followed by those in class IV and less patients were with NYHA Class II HF. In all patients we tested muscle tissue density and all of them did 6MWT(m). The group was spilt by sec. The middle age of the patients in the group was 73±9 years.
Age is an independent factor for sarcopenia. In this sense, the evaluation of muscle tissue density in patients with HF in old age, makes the evaluation of this patients more precise. While weight loss is related to cachexia, it is not always related to sarcopenia (the old-age related loss of muscle mass and force), because the reduction in muscle mass can be masked by proportional increase in adipose tissue. This can hinder the clinical diagnosis of sarcopenia, and that is why proper imaging techniques are required for accessing skeletal muscle tissue density. Methods like DEXA, CT, MRI can be used for evaluating skeletal muscle mass and density. From clinical point of view it is almost impossible to draw a line between sarcopenia and cachexia related muscle waste in the end stages of HF. It should be mentioned, that skeletal muscle tissue loss starts earlier than the loss of adipose tissue in the progression of HF (Table 2).

Our analysis show that 34.3% of the group was with reduced skeletal muscle tissue density. According to the trials, 10-15% of HF patients develop cardiac cachexia – a condition characterized with loss of body weight due to loss of skeletal muscle and adipose tissues. Patients with severe HF develop many histological anomalies in their skeletal muscle - “cardiac skeletal myopatia”.
Results
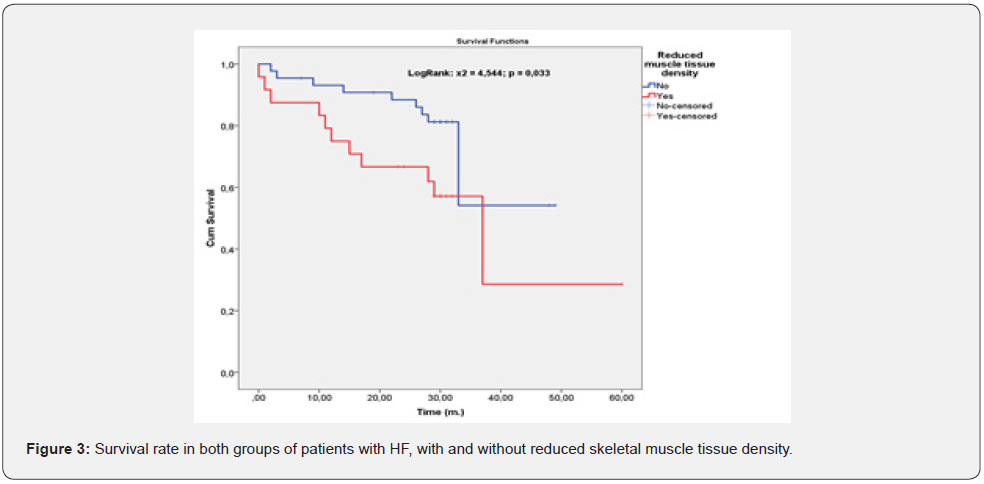
We analyzed the survival rate in the group with reduced muscle density compared to the group without (Figure 3).
We found out that there is significant difference in the survival rate of both groups in favor of the group without sarcopenia. We continued our analysis on the death rate in the same groups (Figure 4). The statistics show that patients with muscle tissue density below 35 H.U. had statistically significant higher death rate in compere to those patients without sarcopenia.

Cardiac cachexia has dramatically progressive impact on patients with HF with 18 moth-to-death probability of 50%. This condition worsens the functional capacity of patients with HF more than it is expected judging only from the evaluation of cardiac dis-function.
The results of our research are similar with the conclusions from the trial “Comparison of Cachexia and Sarcopenia in men with CHF: Results from trials regarding HF worsening co-morbidities (SICA-HF)”. Emami A, Saitoh M, Valentova M at al. found that loss of skeletal muscle with or without body weight loss has higher impact on functional capacity and quality of life among man with CHF.
Our further research prove strong correlative link between sarcopenia and NTproBNP levels (Figure 5). This result show that we should be careful with patients with elevated NTproBNP, which is not only a marker for HF, but it can also be a signal for probable undergoing sarcopenia. Although sarcopenia is a condition mostly dependent by age its progression can be accelerated by simultaneous development of other diseases including HF. The reality is that sarcopenia affects around 20% of older-aged people with HF which is more than frequency of the condition in sameaged people without HF. Older patients with HF and sarcopenia show lower physical capacity that those with HF and preserved muscle mass and function.
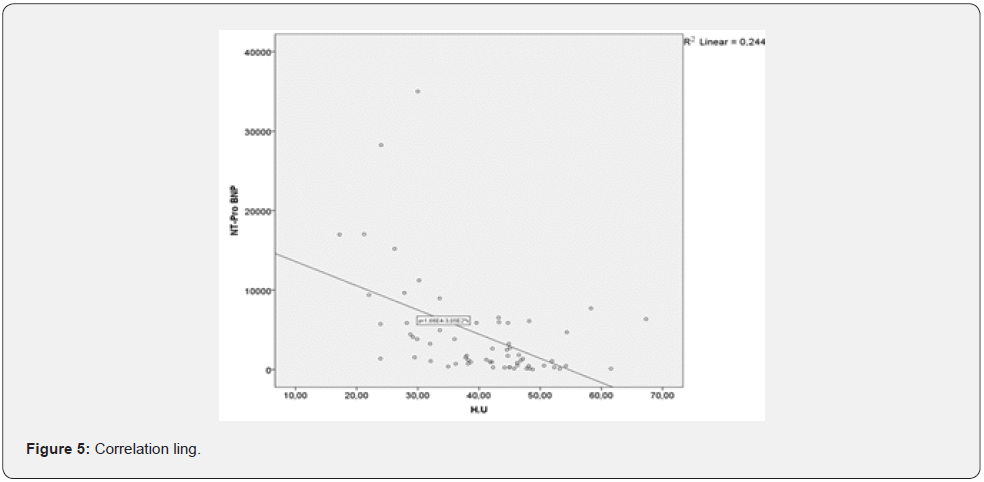
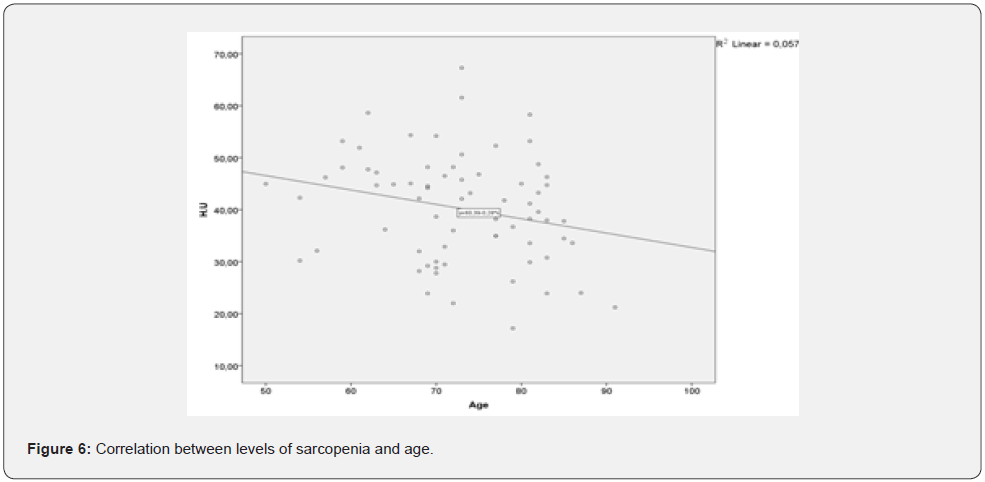
This result show that acknowledging sarcopenia in the context of HF and implementing ad hoc therapeutic strategies can help improving functional capacity of the patients before the HF reaches its later stages. We also concluded that there is strong correlation between skeletal muscle tissue thickness and the age of the HF patients in the observed group (Figure 6) HF and age are synergetic factors, impacting muscle tissue density. That’s why, systematic physical training should have positive effect on patients and even postpone the outcomes of sarcopenia in all patients with HF.
Parallel presence of sarcopenia and HF is often and is probably result from their common pathophysiological pathways such as altered intake and absorbation of nutrients, inflammatory processes, metabolic and autonomic disturbances. These combined processes lead to ultrastructural muscle anomalies, changes in mitochondrial structure and function, increased oxidative stress and change in the distribution of muscle fibers which leads at the end to reduced physical capacity and muscle strength.
Conclusion
In our study sarcopenia proved to be a significant factor in relation to mortality and survival rates of patients with diagnosed HF of all types. Although the group of patients was small, there is a statistical significance and the results of this work point one more direction in treating HF and improving the quality of life of these patients. This filed of HF and cardiology has lot of unknowns by this time. Further studies can give lot of opportunities in the pathophysiological, diagnostic and therapeutic specter of the disease.
References
- Booth FW, Chakravarthy MV, Spangenburg EE (2002) Exercise and gene expression: physiological regulation of the human genome through physical activity. J Physiol 543(Pt 2): 399-411.
- Dutta C, Hadley EC, Lexell J (1997) Sarcopenia and physical performance in old age: overview. Muscle Nerve Suppl 5: S5-S9.
- Booth FW, Chakravarthy MV, Spangenburg EE (2002) Exercise and gene expression: physiological regulation of the human genome through physical activity. J Physiol 543(Pt 2): 399-411.
- Fiatarone MA, Marks EC, Ryan ND, Meredith CN, Lipsitz LA, et al. (1990) High-intensity strength training in nonagenarians. Effects on skeletal muscle. JAMA 263(22): 3029-3034.
- Gonzalez E, Messi ML, Delbono O (2000) The specific force of single intact extensor digitorum longus and soleus mouse muscle fibers declines with aging. J Membr Biol 178(3): 175-183.
- Geffken DF, Cushman M, Burke GL, Polak JF, Sakkinen PA, et al. (2001) Association between physical activity and markers of inflammation in a healthy elderly population. Am J Epidemiol 153(3): 242-250.
- Ferrucci L, Penninx BW, Volpato S, Harris TB, Bandeen-Roche K, et al. (2002) Change in muscle strength explains accelerated decline of physical function in older women with high interleukin-6 serum levels. J Am Geriatr Soc 50(12): 1947-1954.
- Ivey FM, Roth SM, Ferrell RE, Tracy BL, Lemmer JT, et al. (2000) Effects of age, gender, and myostatin genotype on the hypertrophic response to heavy resistance strength training. J Gerontol A Biol Sci Med Sci 55(11): M641-648.
- Ershler WB (1993) Interleukin-6: a cytokine for gerontologists. J Am Geriatr Soc 41(2): 176-181.
- DeVol DL, Rotwein P, Sadow JL, Novakofski J, Bechtel PJ (1990) Activation of insulin-like growth factor gene expression during work-induced skeletal muscle growth. Am J Physiol 259(1 Pt 1): E89-E95.
- Ji LL (2001) Exercise at old age: does it increase or alleviate oxidative stress? Ann NY Acad Sci 928: 236-247.
- Jagoe RT, Goldberg AL (2001) What do we really know about the ubiquitin-proteasome pathway in muscle atrophy? Curr Opin Clin Nutr Metab Care 4(3): 183-190.
- Llovera M, Garcia-Martinez C, Agell N, Lopez-Soriano FJ, Argiles JM (1997) TNF can directly induce the expression of ubiquitin-dependent proteolytic system in rat soleus muscles. Biochem Biophys Res Commun 230(2): 238-241.
- Lynch NA, Metter EJ, Lindle RS, Fozard JL, Tobin JD, et al. (1999) Muscle quality. I. Age-associated differences between arm and leg muscle groups. J Appl Physiol 86(1): 188-194.
- Allen LA, Yager JE, Funk MJ, Levy WC, Tulsky JA, et al. (2008) Discordance between patient-predicted and model-predicted life expectancy among ambulatory patients with heart failure. JAMA 299(21): 2533-2542.






























