Analysis of the factors related to the early outcomes of Type B Acute Aortic Syndromes
Rafael de Athayde Soares1*, Marcelo Fernando Matielo1, Edson Takamitsu Nakamura1, Marcus Vinícius Martins Cury1, Luiz Maurício da Silva Júnior1 and Roberto Sacilotto1
1Department of Vascular/Endovascular Surgery, Instituto de Assistência Médica ao Servidor Público Estadual de São Paulo, Brazil
Submission: September 21, 2021; Published: November 10, 2021
*Corresponding author: Rafael de Athayde Soares, Department of Vascular / Endovascular Surgery, Instituto de Assistência Médica ao Servidor Público Estadual de São Paulo (IAMSPE), São Paulo, SP, Brazil
How to cite this article: Rafael de Athayde S, Marcelo Fernando M, Edson Takamitsu N, Marcus Vinícius Martins C, Luiz Maurício da Silva J, et al. Analysis of the factors related to the early outcomes of Type B Acute Aortic Syndromes. J Cardiol & Cardiovasc Ther. 2021; 17(3): 555962. DOI: 10.19080/JOCCT.2021.17.555962
Abstract
Objective: To determine the outcomes of endovascular treatment (ET) of Type B Acute Aortic Syndrome (AAS) and factors related to mortality and Spinal Cord ischemia.
Methods: This was a retrospective cohort of complicated AAS patients who underwent endovascular treatment. The primary outcome variable was mortality rate and clinical outcomes. The secondary outcome variable was SCI.
Results: Overall, 32 patients were evaluated, 10 patients submitted to CM and 22 patients ET. 100% of the patients had arterial hypertension, with a higher prevalence of male gender (53.1%). There was a higher prevalence of acute aortic dissection Stanford B with 19 cases (59.4%). Among the 22 patients submitted to interventional therapy, most of them thoracic endoprosthesis (TEVAR), with 16 patients (72.7%). A logistic regression showed that refractory chest pain (HR = 9,57; p = 0.005), aortic diameter higher than 40mm (HR = 16.65; p < 0.001), chronic renal failure (CRF) (HR = 5.2; p = 0.009) and SCI (HR = 1.89; p = 0.029) were related to increased mortality rate. Otherwise, male gender (MG) (HR = 17.20; p < 0.001), concomitant TEVAR + EVAR (HR = 3.88; p = 0.048), malperfusion (HR = 3.88; p = 0.038) and number of internal iliac patency (IIP) (HR = 6.52; p = 0.016) were associated to SCI.
Conclusion: This study concluded that refractory chest pain, aortic diameter higher than 40mm, CRF and SCI were related to increased mortality rate. MG, concomitant TEVAR + EVAR, malperfusion and number of IIP were associated to SCI.
Keywords: Acute aortic syndrome; Thoracic aorta; Critical ischemia; Spinal cord ischemia
Introduction
Acute Aortic Syndrome (AAS) includes acute aortic dissection, intra-mural haematoma (IMH), and penetrating aortic ulcer (PAU), which are all characterized by disruption of the medial layer of the aorta and have an inherent risk of imminent aortic rupture [1]. Among these three conditions, aortic dissection is the most common (62-88% of all patients with AAS), followed by IMH (10-30%) and PAU (2-8%) [1-3]. In patients with AAS, 1-year survival is 81% and 5-year survival is 63% [4,5]. The initial management of patients with AAS is focused on the control of blood pressure to reduce aortic wall stress [6].
Acute complicated distal dissection requires urgent invasive treatment and thoracic endovascular aortic repair has become the treatment modality of choice because of favorable outcomes compared to open surgical repair. Intramural hematoma, penetrating aortic ulcers, and traumatic aortic injuries of the descending aorta harbor specific challenges compared to aortic dissection and treatment strategies are not as uniformly defined as in aortic dissection. Moreover, these lesions have a different prognosis [7].
Therefore, the main objective of this study was to assess the outcomes of endovascular treatment of complicated AAS, and the factors related to mortality rate and spinal cord ischemia.
Methods
The study was approved by the Ethical committee for research under the number 3.259.235. All patients treated in our institution consented to the use of anonymized and aggregate data linked to the data basis for the purposes of research. No further patient contact was required. This was a retrospective, consecutive cohort study of patients with AAS who underwent clinical management and / or endovascular repair in the Vascular and Endovascular Surgery Service between January 2011 and December 2018.
Patient data were obtained from the service database using the Microsoft Access® software. The patients’ medical records were also consulted as necessary. Information regarding the surgical procedures was obtained from the service database and the patients medical records.
Patients who were admitted with acute aortic syndrome at the emergency room were included in this present study. All of them were initially submitted to best medical therapy, with aggressive blood pressure control and medications to stabilize the cardiac rate. If necessary, endovenous nitroprusside was administered, in order to best treat the arterial hypertension, which is very common in patients suffering from this condition. In the same way, endovenous metoprolol was also used, in order to stabilize and adequate cardiac rate, to avoid, tachycardia. The patients were stabilized at the emergency room. Once the acute aortic syndrome was suspected, the patients were submitted to an angiotomography, to contribute to the diagnosis of the acute aortic syndrome, and also to evaluate possible severe complications, such as visceral ischemia and aortic ruptures. Patients admitted with uncomplicated acute aortic syndrome were initially treated clinically, without intervention procedures. The only exclusion criteria was aortic dissection Stanford A, due to necessity of cardiovascular surgeon evaluation.
Aortic dissections are classified as complicated or uncomplicated. Complicated dissections are associated with increased mortality owing to poor organ perfusion, aortic rupture or impending rupture, refractory hypertension, persistent pain, expansion >1cm/year, or an overall aortic diameter of >5.5cm. In this cases, endovascular treatment was performed, despite clinical treatment administered initially. On the other hand, Stanford type A Intramural haematoma (IMH) involves the ascending aorta, with or without descending aortic involvement, whereas Stanford type B IMH do not involve the ascending aorta. Patients with IMH that evolved to poor organ perfusion, aortic rupture or impending rupture, refractory hypertension, persistent pain were submitted to endovascular treatment. Patients admitted with PAU were submitted to endovascular treatment in cases of persistent pain, an increase in lesion size, aortic expansion, or rupture [8].
After revisions of the angiotomography and arteriography of the procedures, the patients were classified according to the type of acute aortic syndrome, the type of procedure that was performed in each patient and the diameters of the aorta and anatomical segments.
Regarding the endovascular procedures and spinal cord protection, preoperative insertion of a cerebral spinal fluid drainage catheter is routine for all elective TEVAR procedures. For emergent TEVAR, lumbar drains are placed periperatively as needed if patients develop paraplegia or any signs of spinal cord ischemia. Proximal seal into the aortic dissection is achieved with a thoracic endograft, oversizing the endograft up to 10% of the aortic diameter proximal to the dissected segment in normal aorta, well as having a proximal aortic neck length, or proper landing zone. After deployment, post–balloon dilation is avoided due to the risk of retrograde type A dissection or aortic rupture. When malperfusion is the initial indication, extension of the aortic endograft distally to the celiac artery or selective branch vessel stenting may be necessary if downstream flow remains inadequate. The decision to stent branches is typically based on the clinical, rather than radiologic, severity of malperfusion.
The primary outcome variable was mortality rate and clinical outcomes. The secondary outcome variable were the factors related to spinal cord ischemia.
Statistical analyses were performed using SPSS 15.0 for Windows (SPSS Inc., Chicago, IL, USA). The frequencies of patients and descriptive statistics were calculated. The χ2 test or Student’s t test were used for univariate analyses. Mann-Whitney tests were used for nonparametric variables. Analysis of the variables were made by univariate and multivariate Logistic regression. Statistical significance was defined as a P value of < .05.
Results
Overall, 32 patients with acute aortic syndrome were evaluated. There were 10 patients submitted to clinical treatment, and 22 patients submitted to endovascular treatment. The analyses were performed at admission until hospital discharge, and 30 days post-operative.
The baseline clinical characteristics of the patients are described in Table 1. Regarding the baseline diseases, 100% of the patients had arterial hypertension, with a higher prevalence of male gender (53.1%), Chronic Pulmonary disease (53.1%), tobacco use and diabetes had a prevalence of 40.6%.
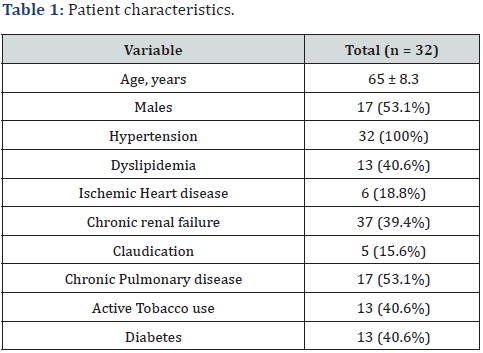
Regarding the type of AAS, there was a higher prevalence of acute aortic dissection Stanford B with 19 cases (59.4%), and the mainly indication for endovascular intervention was intractable chest pain with 87.5%. Furthermore, 6 (18.8%) patients presented with renal impairment, 2 patients (6.3%) with visceral ischemia and 3 patients (9.4%) with limb ischemia. There were two cases of immediate endoleak, 1 endoleak IA and 1 endoleak IB. Both cases were prompt treated with endoprosthesis extension implantation.Those data are summarized on Table 2.
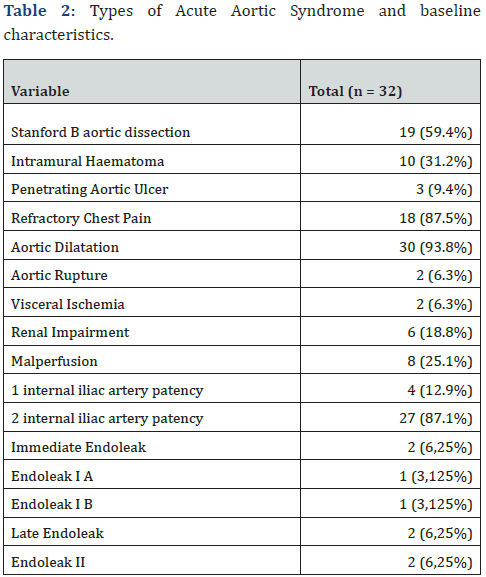
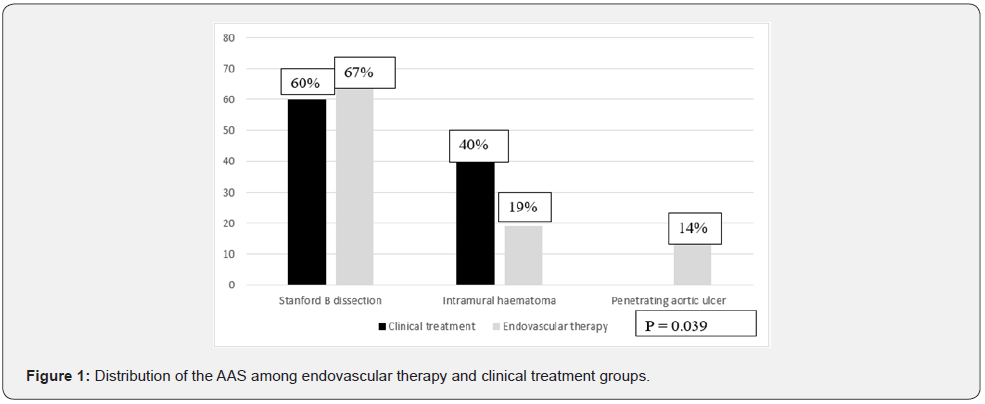
Among the 22 patients submitted to interventional therapy, most of them were submitted to solely thoracic endoprosthesis (TEVAR), with 19 patients (86.4%). Three patients (13.6%) were submitted to both TEVAR and abdominal endoprosthesis (EVAR + TEVAR), due to infra-renal aortic dilatation and abdominal pain, secondary to thoracic aortic dissection. These patients evolved with spinal cord ischemia (2 patients with paraplegia and 1 patient with paraparesis). Seventeen patients (77.3%) had spinal fluid drained for 48hs-72hs. Regarding left subclavian artery, there were 4 patients (18.1%) submitted to left subclavian artery coverage and 3 patients (13.6%) submitted to carotidsubclavian bypass. Those data and the types of endoprosthesis used are summarized on Table 3. Regarding the distribution of the AAS among clinical treatment versus endovascular therapy, there was a higher prevalence of Stanford B dissection and PAU in the endovascular therapy group (p = 0.039) we have those data summarized on Figure 1.
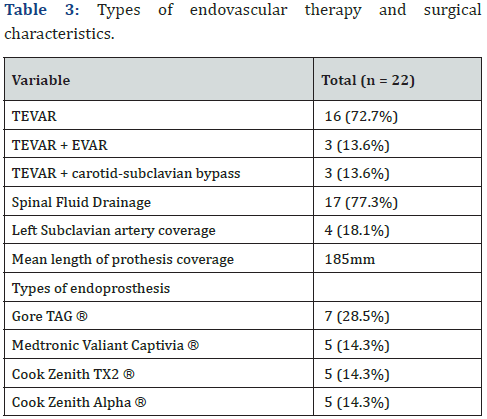
There were 8 patients (36.36%) with post-operative complications. There were 6 patients (27.27%) that evolved with post-operative renal impairment and three patients (13.6%) with spinal cord ischemia. The overall mortality rate was 15.6% (5 patients), 10% in the clinical treatment group and 18.1% in the endovascular therapy group (p = 0.352). Regarding the clinical treatment there was 1 death (10%), due to acute ischemic heart complications. In the endovascular therapy group, there were 4 deaths (18.1%), 1 patient died due to ischemic heart complications, 1 patient with pneumonia and 2 patients died due to acute kidney failure.
The mean diameter of the aortic neck to allow a proximal endoprosthesis implantation was 31.83mm, and the mean length of the aortic neck was 15.97mm. The maximum mean diameter of the thoracic aorta was 50.61mm. Those data are summarized on Table 4.
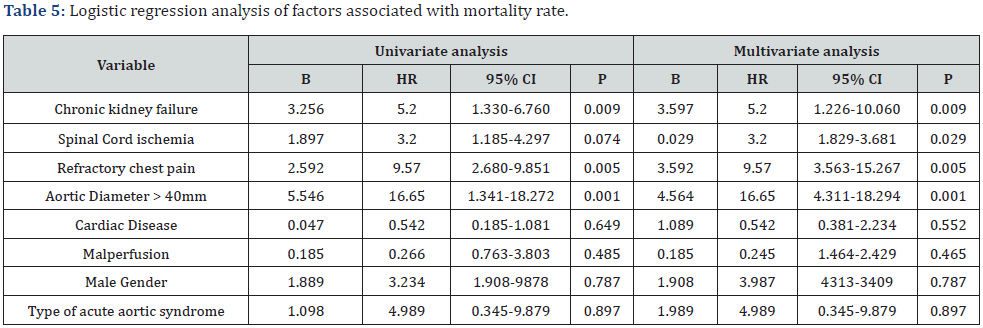
A univariate and multivariate logistic regression was realized in order to evaluate the main factors related to mortality rate. Among several factors tested, refractory chest pain (HR = 9,57; p = 0.005, CI = 3.563–15.267), aortic diameter higher than 40mm (HR = 16.65; p < 0.001, CI= 1.341-18.272), chronic renal failure (HR = 5.2; p = 0.009, CI = 1.330–6.760) and spinal cord ischemia (HR = 1.89; p = 0.029, CI = 1.185–4.297) were related to increased mortality rate (Table 5).

Regarding Spinal cord ischemia, there were 6 cases in the total cohort (18.8%), 3 cases in the clinical treatment group and 3 cases in the endovascular therapy group (30% versus 13.3%; p = 0,086). A univariate and multivariate logistic regression showed that male gender (HR = 17.20; p < 0.001, CI = 1.446–21.760), concomitant TEVAR + EVAR (HR = 3.88; p = 0.048, CI = 1.072– 4.188), malperfusion (HR = 3.88; p = 0.038, CI = 1.442–4.272) and number of internal iliac patency (HR = 6.52; p = 0.016, CI = 1.764- 11.429) were associated to spinal cord ischemia (Table 6).

1 – Concomitant TEVAR + EVAR
2 – Higher than 150mm or < 150mm of aortic coverage.
Discussion
AAS diagnosis and optimal treatment remains a challenging clinical dilemma among vascular emergencies. Failure to recognize AAS, especially in patients without hypertension and typical chest pain, sometimes could explain why this syndrome is reported to be the primary cause of death in these patients [9,10]. In this cohort we present the results regarding patients with AAS admitted in the emergency room, submitted to both clinical therapy and endovascular therapy. Interventional therapy for AAS is indicated for complicated AAS, according to important guidelines [11-13]. Their recommendation class I is that uncomplicated SAA should be treated medically, followed by serial imaging surveillance. Thoracic endovascular aortic repair (TEVAR) should be considered for complicated SAA, recommendation class IIa. TEVAR aims at stabilization of the dissected aorta, to prevent late complications by inducing aortic remodelling processes. Acute type B aortic dissection is the result of a tear in the intimal arterial layer, which allows blood to propagate within the medial layer. This creates a flap, which divides the aorta into a true lumen, and a false lumen. The goal of medical therapy is to reduce systolic blood pressure between 100 and 120mm Hg and, when attainable, the heart rate below 60 beats/min. Two-year follow-up results indicated that TEVAR is effective (aortic remodelling in 91.3% of TEVAR patients vs. 19.4% of patients receiving medical treatment; P < 0.001); however, TEVAR showed no clinical benefit over medical therapy (survival rates: 88.9+3.7% with TEVAR vs. 95.6+ 2.5% with optimal medical therapy; P = 0.15). Extended follow-up of this study (INSTEAD-XL) recently showed that aorta-related mortality (6.9 vs. 19.3%, respectively; P = 0.04) and disease progression (27%.0 vs. 46.1%, respectively; P = 0.04) were significantly lower after 5 years in TEVAR patients compared with those receiving medical therapy only. No difference was found regarding total mortality [11,12]. Similarly in this present study, the indications for endovascular therapy were performed in patients with complicated AAS, due to malperfusion, aortic rupture and other complications, while patients with uncomplicated AAS were submitted to medical therapy.
The most common risk factor associated with AAS is hypertension, observed in 65-75% of individuals, mostly poorly controlled, associated with male gender, with 65% of cases, in the overall literature [13]. In this present study, all patients admitted with AAS had hypertension, poorly controlled and most of then were male. Similarly, among the AAS, there was a higher prevalence of acute aortic dissection Stanford B, followed by intramural haematoma and PAU. Regarding type of AAS in the overall literature, aortic dissection is the most common (62–88% of all patients with AAS), followed by IMH (10-30%) and PAU (2- 8%) [1-3].
Chest pain is the most frequent symptom of SAA in 80% of cases [14]. Abrupt onset of severe chest and/or back pain is the most typical feature. Refractory chest pain consists in one of the most common indications for TEVAR. In this present cohort, refractory chest pain and aortic dilatation were the main indication for TEVAR, followed by malperfusion syndrome and aortic rupture.
Most of patients submitted to endovascular therapy in this present cohort had TEVAR procedure as solely indication, in order to allow the closure of the ‘primary’ entry tear and of perforation sites in the descending aorta. The blood flow is redirected into the true lumen, leading to improved distal perfusion by its decompression. This mechanism may resolve malperfusion of visceral or peripheral arteries. Thrombosis of the false lumen will also be promoted, which is the initiation for aortic remodeling and stabilization. Few cases in this present study required abdominal endoprosthesis implantation as a concomitant procedure, due to abdominal dilatation and malperfusion syndrome. However, these patients evolved with spinal cord ischemia, a catastrophic complication of AAS. There are reports on literature showing spinal cord ischemia in 10-20% of patients with aortic abdominal aneurysm and concomitant thoracic aortic pathologies (type B dissection, penetrating aortic ulcer, thoracic aortic aneurysm) [15]. The increased risk of spinal cord ischemia in patients who require longer segments of aortic coverage, the deployment of multiple stent graft pieces and in patients who have a history of prior abdominal aortic replacement is well described in overall literature [16]. Since spinal cord blood supply is often segmental and dependent on collateral circulation, the need for more extensive aortic coverage interrupts an increasing number of intercostal arteries creating a higher risk of ischemia. Thoracic endovascular aortic repair (TEVAR) in the current era has become the dominant and minimally invasive treatment for type B complicated aorta dissection [17].
In this present cohort, the overall mortality rate was 15.6%, 10% in the clinical treatment group and 18.1% in the endovascular therapy group (p = 0.352). Regarding Spinal cord ischemia, there were 6 cases in the total cohort (18.8%), 3 cases in the clinical treatment group and 3 cases in the endovascular therapy group (30% versus 13.3%; p = 0,086). European registry including 50 patients demonstrated a 30-day mortality of 8% and stroke and spinal cord ischaemia of 8% and 2%, respectively for Stanford B dissection [18]. However, some series showed 30-day mortality rate of 21% [19].
Regarding the factors related to increased mortality rate in this paper, refractory chest pain (HR = 9,57; p = 0.005), aortic diameter higher than 40mm (HR = 16.65; p < 0.001), chronic renal failure (HR = 5.2; p = 0.009) and spinal cord ischemia (HR = 1.89; p = 0.029) were related to worsening prognosis. Among overall literature, the main predictors of IMH complications are persistent and recurrent pain despite aggressive medical treatment, difficult blood pressure control, ascending aortic involvement, maximum aortic diameter ≥50 mm, progressive maximum aortic wall thickness (>11 mm) and detection of organ ischaemia (brain, myocardium, bowels, kidneys, etc) [20,21].
Multiple risk factors for the development of spinal cord ischemia after TEVAR have been identified. Patient risk factors include advanced age (> 70 years old), perioperative hypotension (e.g., mean arterial blood pressure <70 mmHg), renal insufficiency (creatinine >132 umol/L), hypertension, coverage of hypogastric artery, procedure urgency and degenerative aneurysms compared to non-aneurysm pathologies [22,23]. On the other hand, in this present study we have found that male gender (HR = 17.20; p < 0.001), concomitant TEVAR + EVAR (HR = 3.88; p = 0.048), malperfusion (HR = 3.88; p = 0.038) and number of internal iliac patency (HR = 6.52; p = 0.016) were associated to spinal cord ischemia. Malperfusion are related to hypotension and more severity disease, which plays an important factor in the development of spinal cord ischemia. Furthermore, in this present paper, there were 4 patients (18.1%) submitted to left subclavian artery coverage and 3 patients (13.6%) submitted to carotidsubclavian bypass. The patients submitted to left subclavian artery coverage did not present with SCI, with no further complications. According to a recent meta-analysis, there is no significant difference in the rate of SCI between patients with and without left subclavian artery bypass (LSAB) [24]. Futhermore, LSAB itself carries a 10%-12% chance of complications (including brachial plexus, vagus nerve, left recurrent nerve, and thoracic duct injuries; neck hematoma; subclavian dissection; and stroke) [24].
There are some limitations regarding this paper. It is a retrospective analysis, with a not very large number of patients, with a single center experience. However, most of papers about AAS do not have a large simple size. Larger, prospective studies should be performed in order to evaluate the factors related to the outcomes of AAS diagnosis.
Conclusion
This study concluded that refractory chest pain, aortic diameter higher than 40mm, chronic renal failure and spinal cord ischemia were related to increased mortality rate. On the other hand, male gender, concomitant TEVAR + EVAR, malperfusion and number of internal iliac patency were associated to spinal cord ischemia.
References
- Patel PJ, Grande W, Hieb RA (2011) Endovascular management of acute aortic syndromes. Semin. Intervent Radiol 28(1): 10-23.
- Brinster, DR (2009) Endovascular repair of the descending thoracic aorta for penetrating atherosclerotic ulcer disease. J Card Surg 24(2): 203-208.
- LeMaire SA, Russell L (2011) Epidemiology of thoracic aortic dissection. Nat Rev Cardiol 8(2): 103-113.
- Coady MA, Rizzo JA, Elefteriades JA (1999) Pathologic variants of thoracic aortic dissections. Penetrating atherosclerotic ulcers and intramural hematomas. Cardiol Clin 17(4): 637-657.
- Knollmann FD, Lacomis JM, Ocak I, Gleason T (2013) The role of aortic wall CT attenuation measurements for the diagnosis of acute aortic syndromes. Eur J Radiol 82(12): 2392-2398.
- Parker JD, Golledge J (2008) Outcome of endovascular treatment of acute type B aortic dissection. Ann Thorac Surg 86(5): 1707-1712.
- Carpenter SW, Kodolitsch YV, Debus ES, Wipper S, Tsilimparis N, et al. (2014) Acute aortic syndromes: definition, prognosis and treatment options. J Cardiovasc Surg (Torino) 55(2 Suppl 1): 133-144.
- Clough RE, Nienaber CA (2015) Management of acute aortic syndrome. Nat Rev Cardiol 12(2): 103-114.
- Alfson DB Ham SW (2017) Type B aortic dissections: current guidelines for treatment. Cardiol Clin 35(3): 387-410.
- Landenhed M, Engström G, Gottsäter A, Caulfield MP, Hedblad B, et al. (2015) Risk profiles for aortic dissection and ruptured or surgically treated aneurysms: a prospective cohort study. J Am Heart Assoc 246.
- Nienaber CA, Rousseau H, Eggebrecht H, Kische S, Fattori R, et al. (2009) Randomized comparison of strategies for type B aortic dissection: the INvestigation of STEnt Grafts in Aortic Dissection (INSTEAD) trial. Circulation 120(25): 2519-2528.
- Erbel R, Aboyans V, Boileau C, Bossone E, Di Bartolomeo R, et al. (2014) 2014 ESC Guidelines on the diagnosis and management of aortic disease Eur Heat J 35(41): 2873-2926.
- Riambau V, Bockler D, Brunkwall, Cao P, Chiesa R, et al. (2017) ESVS Guidelines on thoracic aorta in Management od thoracic aortic disease. Eur J Vasc Endovasc Surg 53(1): 4-52.
- Moro H, Hayashi J, Sogawa M (1999) Surgical management of the ruptured aortic arch. Ann Thorac Surg 67(2): 593-594.
- Moon MR, Mitchell RS, Dake MD, Zarins CK, Fann JI, et al. (1997) Simultaneous abdominal aorti replacement and thoracic stent graft placement for multilevel aortic disease. J Vasc Surg 25(2): 332-340.
- Carroccio A, Marin ML, Ellozy S, Hollier LH (2003) Pathophysiology of paraplegia following endovascular thoracic aortic aneurysm repair. J Card Surg 18(4): 359-366.
- Zeng Q, Guo X, Huang L, Sun L (2017) Single-center experience with simultaneous thoracic endovascular aortic repair and abdominal endovascular aneurysm repair. Vascular 25(2): 157-162.
- O’Gara PT, DeSanctis RW (1995) Acute aortic dissection and its variants. Toward a common diagnostic and therapeutic approach. Circulation 92(6): 1376-1378.
- Dake MD, Kato N, Mitchell RS, Semba CP, Razavi MK, et al. (1999) Endovascular stent-graft placement for the treatment of acute aortic dissection. N Engl J Med 340(20): 1546-1552.
- Ganaha F, Miller DC, Sugimoto K, Do YS, Minamiguchi H, et al. (2002) Prognosis of aortic intramural hematoma with and without penetrating atherosclerotic ulcer: a clinical and radiological analysis. Circulation 106(3): 342-348.
- Evangelista A, Dominguez R, Sebastia C, Salas A, Permanyer-Miralda G, et al. (2004) Prognostic value of clinical and morphologic findings in short-term evolution of aortic intramural haematoma. Therapeutic implications. Eur Heart J 25(1): 81-87.
- Ullery BW, Cheung AT, Fairman RM, Jackson BM, Woo EY, et al. (2011) Risk factors, outcomes, and clinical manifestations of spinal cord ischemia following thoracic endovascular aortic repair. J Vasc Surg 54(3): 677-684.
- Scali ST, Wang SK, Feezor RJ, Huber TS, Martin TD, et al. (2014) Preoperative prediction of spinal cord ischemia after thoracic endovascular aortic repair. J Vasc Surg 60(6): 1481-1490.e1.
- Awad H, Ramadan ME, El Sayed HF, Tolpin DA, Tili E, et al. (2017) Spinal cord injury after thoracic endovascular aortic aneurysm repair. Can J Anaesth 64(12): 1218-1235.






























