Kissing Intravascular Balloon Lithoplasty and Endovascular Aortic Repair as a Treatment of Severe Distal Aorto-Iliac Occlusive Disease to Ensure a Safe Conduit to Facilitate Thoracic Endovascular Aortic Repair
Pankaj Khullar1*, Joshua Berookhim2, Justin Ratcliffe1, Reid Ravin3, Gabriele Di Luozzo4, Naveed Rajper1 and Joseph Puma1
1Department of Cardiovascular at Mount Sinai Morningside Hospital, New York, NY, USA
2Department of Medicine at Mount Sinai Morningside and Mount Sinai West, New York, NY, USA
3Department of Vascular Surgery at Mount Sinai Morningside Hospital, New York, NY, USA
4Department of Cardiothoracic Surgery at Mount Sinai Morningside Hospital, New York, NY, USA
Submission: October 08, 2020;Published: November 20, 2020
*Corresponding author: Pankaj Khullar, Department of Cardiovascular at Mount Sinai Morningside Hospital, New York, NY, USA, 8718 Bay Parkway Fl 1 and 2, Brooklyn, NY 11214
How to cite this article:Pankaj K, Joshua B, Justin R, Reid R, Gabriele D L, et al. Kissing Intravascular Balloon Lithoplasty and Endovascular Aortic Repair as a Treatment of Severe Distal Aorto-Iliac Occlusive Disease to Ensure a Safe Conduit to Facilitate Thoracic Endovascular Aortic Repair. J Cardiol & Cardiovasc Ther.2020; 16(4): 555943. DOI: 10.19080/JOCCT.2020.16.555943
Abstract
Background: We describe a novel case of treating severe distal aorto-illiac occlusive disease and endovascular aortic repair with kissing endoluminal lithoplasty balloons. This ensured a safe conduit to facilitate thoracic endovascular aortic repair.
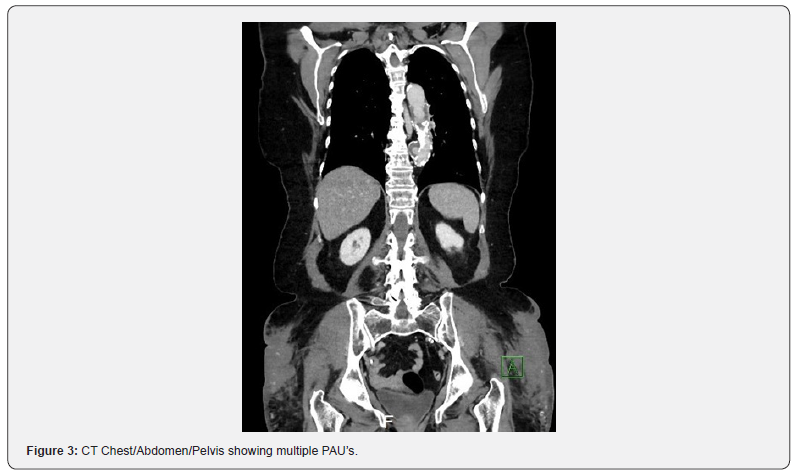
Case Presentation: A patient with multiple comorbidities including coronary artery disease and peripheral artery disease (Rutherford 4 symptoms bilaterally) presented to the emergency room with chest pain and dyspnea on exertion. The patient was found to have a 3.5 cm descending thoracic aortic aneurysm, multiple penetrating aortic ulcers (PAU’s) within the descending thoracic aorta which was felt to be the cause of her chest pain along with heavily calcified occlusive aorto-iliac disease. The patient was deemed high surgical risk for open repair and the patient’s severe calcific distal aortic stenosis and bilateral severe ostial iliac stenosis was deemed a barrier for endovascular repair. Therefore, the patient was successfully treated with kissing balloon lithoplasty for lesion preparation of the heavy calcification and placement of an endovascular stent graft (EVAR) in the distal aorta, which ensured a safe conduit for thoracic endovascular aortic repair (TEVAR) procedure.
Conclusion: As patients become more and more complex it is important to explore endovascular treatment options especially when surgical risk is high. Balloon lithoplasty and graft placement of calcified aorto-iliac bifurcation is a safe approach to develop a clear conduit for TEVAR.
Keywords: Aneurysm; Peripheral artery disease; Peripheral vascular disease; Endovascular treatment/therapy; Penetrating aortic ulcer; Thoracic aortic aneurysm; Aortoiliac bifurcation; Stenosis; Kissing Endoluminal Lithoplasty
Abbreviations: PAU’s: Penetrating Aortic Ulcers; EVAR: Endovascular Aortic Repair; TEVAR: Thoracic Endovascular Aortic Repair; RCA: Right Coronary Artery; CFA: Common Femoral Artery; CIA: Common Iliac Artery
Background
Surgical intervention, endovascular repair, and medical management constitute the three treatment options for patients with acute aortic syndromes including: thoracic or thoracoabdominal aneurysms, PAU’s, or aortic dissections. Symptomatic acute aortic syndromes are usually treated but asymptomatic or uncomplicated type B aortic dissection or acute intra-mural hematoma may be managed conservatively [1-3]. Surgical interventions require thoracic or thoracoabdominal incisions and aortic cross-clamping and can be avoided with an endovascular approach. Endovascular repair has other benefits which include reduced perioperative morbidity and mortality, less blood loss, decreased perioperative pain, diminished respirator dependency, and diminished incidence of visceral, renal, and spinal cord ischemia [4-6]. Endovascular approach however is not always an option due to unfavorable anatomy, lack of vascular access options, and heavily calcification of the aorto-iliac vessels which makes the chance of procedural success less likely. However, proper lesion preparation can exponentially increase your chance of procedural success and a favorable outcome for the patient.
Case Presentation
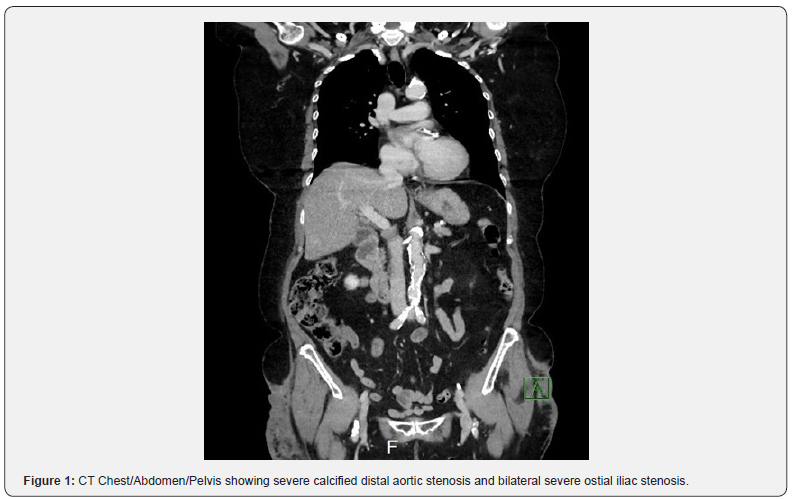
73-year-old female with past medical history of non-insulin dependent diabetes mellitus, hypertension, hyperlipidemia, coronary artery disease (stent to the distal and mid right coronary artery [RCA]), and peripheral artery disease (Rutherford 4 symptoms bilaterally) presented to the emergency department for sudden onset chest pain and dyspnea on exertion. Patient had multiple previous admissions with similar complaints for chest pain and had multiple biochemical assays and imaging studies including coronary angiography to evaluate for coronary artery disease, which were negative. During this admission, the patient underwent CT angiography which demonstrated a 3.5 cm descending thoracic aortic aneurysm, multiple PAU’s within the descending thoracic aorta, a severely calcified distal aortic stenosis and bilateral severe ostial iliac stenosis (Figures 1-3). Given recurrent admissions and negative cardiac work up, the thoracic penetrating aortic ulcer was thought to be the etiology of her chest pain. There was concern regarding the treatment of the thoracic penetrating ulcer as the patient was ultimately deemed high-risk for surgical repair and there was no clear conduit to get to the thoracic aorta by an endovascular approach due to the severe distal aorto-iliac occlusive disease. After discussions between vascular surgery and interventional cardiology, a decision was made to proceed initially to treat the severe distal aorto-iliac stenosis with a bifurcated stent graft to ensure a clear endovascular passage to ultimately treat the thoracic aortic disease. Calcium, plaque modification and possible stenting was deemed a necessary and warranted strategy for lesion preparation to help facilitate the large TEVAR delivery system. Lack of proper lesion preparation of heavy calcificed lesions can lead to complications such as dissection, perforation, inability to advance the device, and ultimately failure of the procedure. Intravascular balloon lithoplasty has shown favorable results in recent clinical experience in the realm of structural heart disease in order to complete transcatheter aortic valve replacement (TAVR) procedures therefore this method of lesion preparation was chosen for this case [7,8]. Intravascular lithoplasty functions by incorporating lithoplasty emitters within an angioplasty balloon, such that sonic pressure waves drive through surrounding tissue selectively fracturing vascular calcium within the vessel wall, thus purportedly altering vessel compliance and permitting vessel dilation at relatively low pressures. Other methods to prepare the lesions such as balloon angioplasty or atherectomy were considered but was believed that it was inadequate and may leave residual stenosis and unsatisfactory result within the artery.


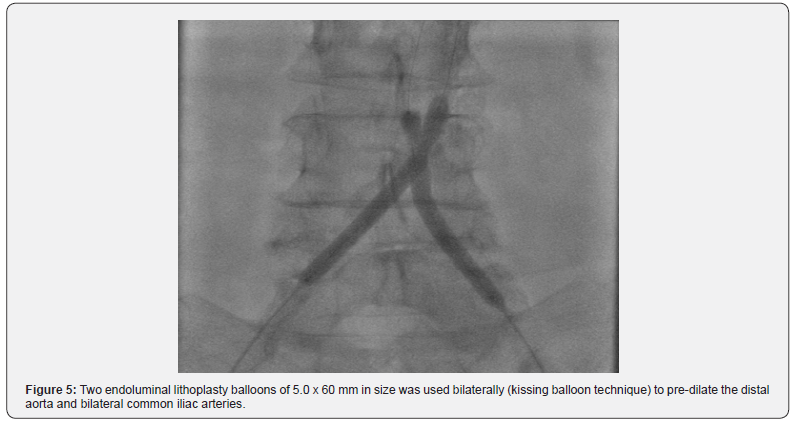
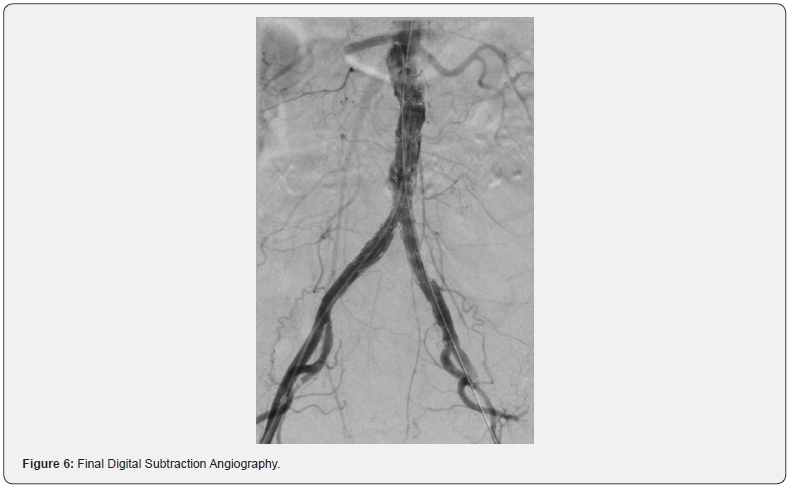
Bilateral percutaneous access to the right and left common femoral arteries was obtained using standard techniques. Angiography was performed to measure the vessel length and characterize the anatomy (Figure 4). Two intravascular lithoplasty balloons (Shockwave Medical, Santa Clara, CA) of 5.0 x 60 mm in size was used bilaterally (kissing balloon technique) to predilate the distal aorta and bilateral common iliac arteries (Figure 5). The kissing lithoplasty was followed by a single 7.0 x 40 mm endoluminal lithoplasty balloon to predilate the ostium left common iliac artery (CIA) and distal aorta to advance the main limb of the graft. An AFX 22x70/iliac 16-30 bifurcated device (Endologix, Irvine, Ca) was then advanced into the distal aorta without significant resistance and the main body and both contralateral and ipsilateral limbs were deployed [9]. Post dilatation with two 7.0 x 40mm balloons were performed in a kissing balloon fashion and individually in both limbs. A final angiogram showed successful deployment and expansion of the graft with no residual lesion identified (Figure 6). The catheters and sheaths were then removed, and vascular access hemostasis was obtained.
The patient was monitored overnight and discharged home the following day without complications. The patient returned one month later for staged TEVAR. A 30 x 200 mm Cook Zenith Alpha stent graft (Cook Medical, Bloomington, IN) with a 16 French delivery system was used. Patient was seen for multiple follow up visit post procedure and the patient no long had any chest pain.
Conclusion
PAU’s if not corrected can lead to intramural hemorrhage, pseudoaneurysm formation, and ultimately transmural aortic rupture [10]. In this case report, the authors describe a novel case of the use of kissing balloon lithoplasty of a severe calcified distal aorto-ostial iliac stenosis to help facilitate the placement of endovascular stent graft. Heavy calcification in the aortoiliac bifurcation makes delivery of TEVAR device very difficult. Heavy calcification also puts the patient at risk of developing complications such as dissection, perforation, and failure to complete the procedure. Kissing balloon lithoplasty has recently been used as a novel new technique in the other percutaneous procedures necessitating large bore access and devices which makes calcified lesions easier to treat. Both clinical and experimental studies have shown that staging aortic stenting or replacement reduces the incidence of spinal cord ischemia. In a porcine experimental model, the incidence of paraplegia nears zero if these interventions are staged [11-13]. Given this patient’s diseased hypogastric arteries, a major conduit to spinal cord perfusion, and the amount of aortic coverage with both EVAR and TEVAR, a stage approach was deemed prudent to reduce the risk of spinal cord ischemia.
As patients become more and more complex, it is important to explore novel endovascular treatment options to facilitate thoracic aneurysm repairs especially when surgical risk is high.
Declarations
• Ethics approval and consent to participate: this was a case report and the need for approval was waived.
• Consent for publication: written consent obtained from patient.
• Availability of data and material: Not applicable. If your manuscript does not contain any data, please state ‘Not applicable’ in this section.
Competing Interests
The authors declare that they have no competing interests.
Funding:
No funding involved with this case report.
Authors’ contributions:
PK: assisted with the intervention and prepared the manuscript. JB: prepared the manuscript. JR: assisted with the intervention. RR: assisted with the intervention. GDL: assisted with the intervention. NR: assisted with the intervention. JP: assisted with the intervention.
References
- Clift PF, Cervi E (2020) A review of thoracic aortic aneurysm disease. Echo Res Pract7(1):R1-R10.
- Murphy MC, Castner CF, Kouchoukos NT (2017) Acute Aortic Syndromes: Diagnosis and Treatment. Mo Med114(6):458-463.
- Tsai TT, Nienaber CA, Eagle KA (2005) Acute aortic syndromes. Circulation112(24):3802-3813.
- Clare R, Jorgensen J, Brar SS (2016) Open Versus Endovascular or Hybrid Thoracic Aortic Aneurysm Repair. CurrAtheroscler Rep18(10):60.
- Kärkkäinen JM, Pather K, Tenorio ER, Mees B, Oderich GS (2019) Should endovascular approach be considered as the first option for thoraco-abdominal aortic aneurysms? J Cardiovasc Surg (Torino)60(3):298-312.
- Kawaharada N, Morishita K, Fukada J,Hachiro Y, Fujisawa Y, et al. (2005) Stroke in surgery of the arteriosclerotic descending thoracic aortic aneurysms: influence of cross-clamping technique of the aorta. Eur J Cardiothorac Surg27(4):622-625.
- Di Mario C, Chiriatti N, Stolcova M, Meucci F, Squillantini G (2018)Lithoplasty-assisted transfemoral aortic valve implantation. Eur Heart J.
- Di Mario C, Goodwin M, Ristalli F,Ravani M, Meucci F, et al. (2019) A Prospective Registry of Intravascular Lithotripsy-Enabled Vascular Access for Transfemoral Transcatheter Aortic Valve Replacement. JACC Cardiovasc Interv12(5):502-504.
- Maldonado TS, Westin GG, Jazaeri O,Mewissen M, Reijnen MMPJ, et al. (2016) Treatment of Aortoiliac Occlusive Disease with the Endologix AFX Unibody Endograft. Eur J VascEndovasc Surg52(1):64-74.
- D'Annoville T, Ozdemir BA, Alric P, Marty-Ane CH, Canaud L (2016) Thoracic Endovascular Aortic Repair for Penetrating Aortic Ulcer: Literature Review. Ann Thorac Surg101(6):2272-2278.
- Bischoff MS, Brenner RM, Scheumann J,Zoli S, Di Luozzo G, et al. (2012) Staged approach for spinal cord protection in hybrid thoracoabdominal aortic aneurysm repair. Ann Cardiothorac Surg1(3):325-328.
- Etz CD, Zoli S, Mueller CS,Bodian CA, Di Luozzo G, et al. (2010) Staged repair significantly reduces paraplegia rate after extensive thoracoabdominal aortic aneurysm repair. J Thorac Cardiovasc Surg139(6):1464-1472.
- Zoli S, Etz CD, Roder F,Brenner RM,Bodian CA,et al. (2010) Experimental two-stage simulated repair of extensive thoracoabdominal aneurysms reduces paraplegia risk. Ann Thorac Surg90(3):722-729.






























