The MicroRNA-210/Casp8ap2 Pathway Alleviates Hypoxia-Induced Injury in Myocardial Cells by Regulating Apoptosis and Autophagy
Kunsheng Li1,2, Jun Pan1, Qiuchang Li3, Shiliang Li6, Kai Li1, Yongqing Cheng1, Lin Chai1, Chao Li4, Junling Li3, Zhikun Fu5, Dongjin Wang1* and Yang Bai7*
1Department of Cardiothoracic Surgery, Nanjing Drum Tower Hospital, The Affiliated Hospital of Nanjing University Medical School, 321 Zhongshan Road, Nanjing 210008, Jiangsu Province, P.R. China
2Department of Cardiac Surgery, University of Heidelberg, 69120 Heidelberg, Germany
3Puyang Medical College, Shangyang Road and Wenyan Street, Puyang 457000, Henan Province, P.R. China
4School of life sciences, Anhui Medical University, 81 Meishan Road, Hefei 230000, Anhui Province, P.R. China
5Department of Cardiac Surgery, The 7th people’s hospital of Zhengzhou, 17 Jingnan wu Road, Zhengzhou 450000, Henan Province, P.R. China
6Department of Cardiac Surgery, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430030, Hubei Province, P.R. China
7Division of Cardiology, Department of Internal Medicine, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430030, Hubei Province, P.R. China
Submission: June 26, 2020; Published: July 21, 2020
*Corresponding author: Dr. Yang Bai, Division of Cardiology, Department of Internal Medicine, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Jiefang Road, No 1095, Wuhan 430030, Hubei Province, P.R. China Dr. Dongjin Wang, Department of Cardiothoracic Surgery, Nanjing Drum Tower Hospital, The Affiliated Hospital of Nanjing University Medical School, 321 Zhongshan Road, Nanjing 210008, Jiangsu Province, P.R. China/p>
How to cite this article:Kunsheng L, Jun P, Qiuchang L, Shiliang L, Kai L, et al. The MicroRNA-210/Casp8ap2 Pathway Alleviates Hypoxia-Induced Injury in Myocardial Cells by Regulating Apoptosis and Autophagy. J Cardiol & Cardiovasc Ther. 2020; 16(3): 555936. DOI: 10.19080/JOCCT.2020.16.555937
Abstract
Aim: This study was conducted to investigate the role of the miR-210/Caspase8ap2 pathway in apoptosis and autophagy in hypoxic myocardial cells.
Methods: The miR-control, miR-210 mimic and miR-210 inhibitor were transfected into rat myocardial H9C2 cells. The transfection efficiency of exogenous miR-210 was determined by quantitative reverse-transcription polymerase chain reaction (qRT-PCR). H9C2 cells were then treated with CoCl2 for 24, 48 and 72 h to generate a myocardial injury model. The apoptosis of H9C2 cells was assessed by flow cytometry. Additionally, a western blot assay was used to determine the expression of the autophagy-associated proteins light chain 3 (LC3), p62 and Beclin-1, and apoptosis-associated proteins Caspase8ap2, cleaved caspase 8 and cleaved caspase 3.
Results: We determined that a 48 h hypoxia treatment duration in H9C2 cardiomyocytes induced myocardial injury. Additionally, the overexpression of miR-210 significantly inhibited cell apoptosis. MiR-210 suppressed autophagy by upregulating p62 and downregulating LC3II/I in hypoxic H9C2 cells. Since Caspase8ap2 was a putative target of miR-210, miR-210 mediated apoptosis and autophagy of H9C2 cells via suppressing Caspase8ap2. Furthermore, the expression of caspase 8, caspase 3 and Beclin-1 were decreased in response to miR-210.
Conclusions:miR-210 exhibits anti-apoptosis and anti-autophagy effects, which alleviate myocardial injury in response to hypoxia.
Keywords: MiR-210; Caspase8ap2; Myocardial injury; Hypoxia; Apoptosis; Autophagy
Introduction
Coronary heart disease (CHD) is characterized by myocardial ischemia, anoxia and necrosis attributed to coronary atherosclerosis, which results in heart defects. CHD is a leading cause of death and disability, with a 10-year mortality rise of 60% in China [1,2]. This devastating rise is mainly due to adverse trends in risk factors, such as blood pressure, cholesterol and diabetes [3]. Identifying mechanisms to inhibit myocardial cell apoptosis under a hypoxic-ischemic state is a recent topic of high interest in CHD research.
Many studies have shown that microRNAs (miRNAs), an abundant class of endogenic non-coding RNAs with approximately 22 nucleotides, are involved in a wide variety of biological processes by modulating the expression of target genes and pathways [4,5]. For CHD, an increasing number of miRNAs have been identified that play considerable roles in CHD development, such as miR-320b [6], miR-224 [7], miR-423, miR-208a and miR-1 [8].
Among various miRNAs, miR-210 plays a significant role in the hypoxia response on the account of its high sensitivity to hypoxia. Recent evidence has emerged that suggests miR-210, as the main hypoxia-induced miRNA, has pivotal implications in cell survival and angiogenesis [9]. During hypoxia stress, miR- 210 was potently upregulated in myocardial cells in vitro [10]. In addition, targeted miR-210 could effectively protect the heart from myocardial infarctions and promote functional recovery [11]. However, little is known about the mechanism of miR-210 in ischemia-hypoxia in myocardial cells.
Caspase-8-associated protein-2 (Caspase8ap2) consists of 1926 amino acids and is an integral member of the apoptosis signalling complex that activates caspase-8 and downstream caspase 3 by combining with the death receptor of procaspase-8 [12]. Thus, Caspase8ap2 is considered a pro-apoptosis protein. Kim et al. [12] reported that the apoptosis in mesenchymal stem cells (MSCs) caused by ischemic preconditioning (IP) was suppressed by miR-210 by repressing Caspase8ap2 [12].
The present study was conducted to investigate the role of the miR-210/Caspase8ap2 pathway in apoptosis and autophagy in hypoxic myocardial cells. The findings of this study can potentially provide novel avenues for the treatment of myocardial injury induced by hypoxia.
Materials and Methods
Hypoxic myocardial injury model
The rat myocardial cell line H9C2 (American Type Culture Collection, Manassas, USA) was cultured in Dulbecco’s Modified Eagle’s Medium (DMEM, Gibco-BRL, Grand Island, USA) supplemented with 10% fetal bovine serum (FBS; HyClone, Logan, USA). All cells were maintained in a humidified incubator containing 95% air and 5% CO2 at 37°C. Cells were cultured to 70-80% confluence, which was determined using a phase contrast microscopy (Olympus, Tokyo, Japan). After reaching 70%-80% confluence, H9C2 cells were treated with 400 μM CoCl2 for 24, 48 and 72 h.
Cell transfection
Hypoxic H9C2 cells were seeded at a density of 3 × 104 cells/ well in six-well plates and allowed to adhere to the dish overnight. The next day, the miR-negative control (NC), miR-210 mimic and miR-210 inhibitor (GenePharma Co, Shanghai, China) were transfected into H9C2 cells using Lipofectamine 2000 reagent (Life Technologies, Gaithersburg, USA) according to manufacturer’s protocol.
Quantitative reverse-transcription polymerase chain reaction (qRT-PCR)
Total RNA was extracted from normal H9C2 cells, hypoxic H9C2 cells, NC, miR-210 mimic and miR-210 inhibitor group with TRIzol reagent (Invitrogen, Carlsbad, USA) following the manufacturer’s instructions. Briefly, single-stranded complementary DNA (cDNA) was synthesized from 2 μg of RNA via the RevertAid First Strand cDNA Synthesis Kit (Fermentas, Ontario, Canada). Subsequently, the generated miR-210 cDNA was amplified via qRT-PCR based on the TaqMan microRNA assay protocol (Roche LightCycler 480 II System). Briefly, 20 μL reactions were incubated in a 96-well optical plate at 94°C for 2 min and then subjected to 40 cycles of 94°C for 20 s and 60°C for 34 s. The relative expression of miR- 210 was normalized to the internal control (U6) using the 2–ΔΔCt method. The primers for miR-210 and U6 were synthesized with the miScript Primer Assay kit (Qiagen, Dusseldorf, Germany).
Apoptosis assay
Apoptotic cells in NC, miR-210 mimic and miR-210 inhibitor groups were quantified using the Annexin V-FITC apoptosis detection kit (Beyotime Institute of Biotechnology, Jiangsu, China). Both the attached cells and cells in the supernatant were collected from the above four groups and fixed with 2% paraformaldehyde. They were washed twice with cold phosphate-buffered saline (PBS) and resuspended in 100 μl of the annexin-binding buffer. Then, H9C2 cells were incubated with 2.5 μl of Annexin V for 15 min and analysed using an EPICS XL flow cytometer (Beckman- Coulter, Fullerton, USA). Apoptotic cells were defined as the cells that belonged to the right quadrant (right top + right bottom) in flow cytometry plots. The experiment was independently repeated three times, and an average apoptotic rate was calculated.
Western blot
Standard western blotting was conducted for protein expression assays in hypoxic H9C2 cells transfected with miR- 210 mimic, inhibitor and NC. Two days following transfection, proteins were isolated with RIPA lysis buffer containing 1 mg protease inhibitors (Applygen Technologies Inc., Beijing, China). The protein contents were quantified using the Bicinchoninic Acid (BCA) Protein Assay Kit (CoWin Biotech Co., Ltd., Beijing, China). Primary antibodies against light chain 3 (LC3; ab48394), p62 (ab56416), Caspase8ap2 (ab4052), cleaved caspase 8 (ab25901), cleaved caspase 3 (ab13847), Beclin-1 (ab62557), and the internal controls b-actin and GAPDH were from Abcam (Cambridge, UK). Furthermore, secondary antibodies labelled with horseradish peroxidase were incubated with membranes for 1 h at 37°C. Samples were then transferred to a polyvinylidene fluoride (PVDF) microporous membrane (Millipore, Boston, USA). Blots were imaged by a FluorChem E imager (Cell Biosciences, San Jose, USA). The grey value of each band was determined with ImageJ version 1.51.
Luciferase reporter assay
For transfections, host H9C2 cells were plated in triplicate into 24-well plates containing Lipofectamine 2000 (Invitrogen, Carlsbad, USA) and 0.8 μg pEZX-miR-210 (or pEZX-miR-Sc) per well. The precursor miR-210 expression clone was established via a feline immunodeficiency virus using the lentiviral vector system (pEZX-miR-210). Subsequently, we designed the luciferase reporter targeting the 3-UTR of Caspase8ap2 to surround the binding sites of miR-210. The Dual-Luciferase Reporter Assay System kit (Promega, Madison, USA) was used to measure the amount of luciferase and Renilla luciferase activity with a fluorescence spectrophotometer. The relative transcriptional activity was normalized to its corresponding value.
Statistical analysis
All data were expressed as the mean ± standard deviation (SD). Statistical differences were evaluated by SPSS 19.0 software. Statistical analysis was performed using a one-way analysis of variance and Student’s t-test, and a P-value < 0.05 was considered as statistically significant.
Results
The expression of miR-210 in H9C2 was upregulated in hypoxic H9C2 cells
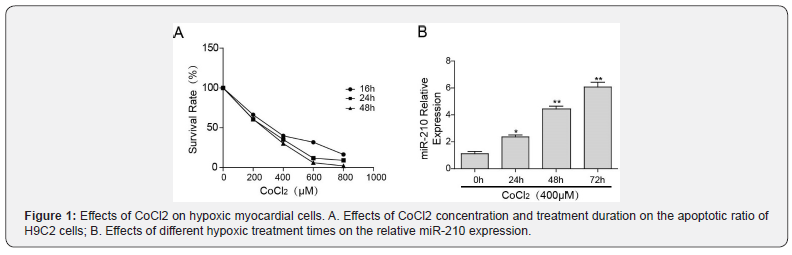
To generate an optimized model of anoxic myocardial cells, H9C2 cells were treated with CoCl2 for 24, 48 and 72 h. The number of apoptotic cells and relative miR-210 expression are shown in Figure 1. With increasing CoCl2 concentrations and treatment duration, the apoptosis ratio of H9C2 cells was enhanced (Figure 1A). Additionally, the relative expression of miR-210 increased in a time-dependent manner (Figure 1B). We found that a 24 h and 48 h hypoxia treatment provoked similar apoptosis ratios in H9C2 cells. Therefore, a 48 h hypoxia duration was used in H9C2 cardiomyocytes for all subsequent experiments.
Overexpressing miR-210 inhibited cell apoptosis in hypoxic H9C2 cells
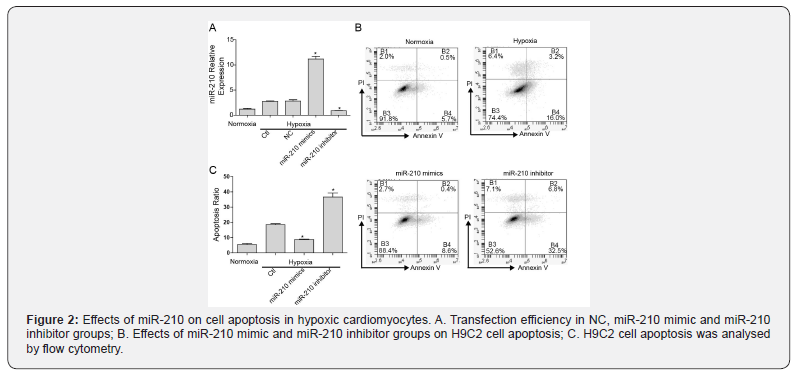
We evaluated the effects of miR-210 overexpression on cell apoptosis in hypoxic cardiomyocytes by generating NC, miR-210 mimic and miR-210 inhibitor groups under hypoxia culture for 48 h. The relative expression of miR-210 was potently enhanced in the miR-210 mimic group but was suppressed in the miR- 210 inhibitor group, which demonstrated the high transfection efficiency in H9C2 cells (Figure 2A). Moreover, there was no significant difference in the apoptosis ratio between hypoxia and NC groups (data not shown). Overexpressing miR-210 caused a decline in apoptosis, whereas silencing miR-210 had the opposite effect. It was uncovered that overexpressing miR-210 inhibited cell apoptosis in hypoxic H9C2 cells (Figure 2B and C).
Overexpressing miR-210 inhibited autophagy in hypoxic H9C2 cells
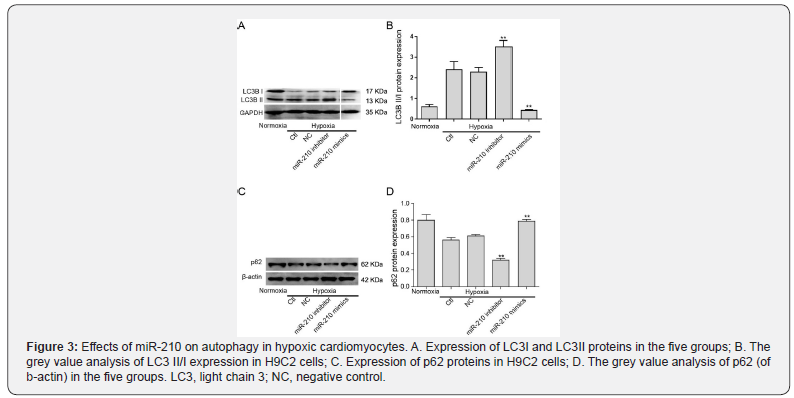
Next, the expression of autophagy-associated proteins LC3 and p62 were assessed by western blot to investigate the effects of miR-210 overexpression on autophagy in hypoxic H9C2 cells. As shown in Figure 3, LC3 II/I was upregulated and p62 was downregulated in the hypoxia group compared with the normoxia group, which demonstrated that there was a certain degree of autophagy in H9C2 cells. Furthermore, the expression of LC3 II/I was significantly lower, and the expression of p62 was higher in the miR-210 mimic group compared with other groups, which indicated that miR-210 overexpression inhibited autophagy in H9C2 cells under hypoxic conditions.
Caspase8ap2 was a target gene of miR-210
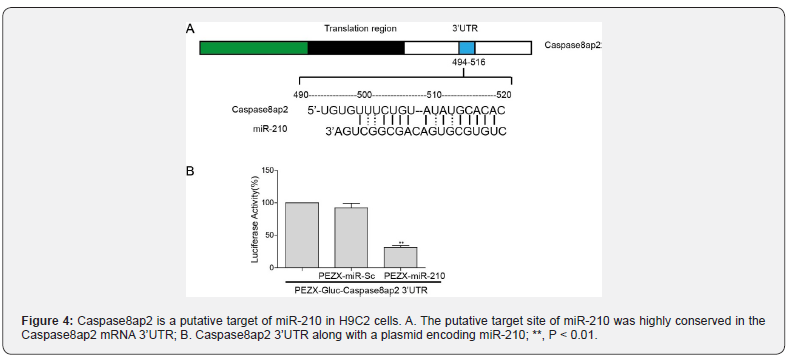
To explore whether Caspase8ap2 was a target gene of miR- 210, we performed a dual-luciferase reporter gene assay in H9C2 cells. Our computational studies identified consensus putative target sites of miR-210 with high complementarity relevant to the 3’UTR region of Caspase8ap2 (Figure 4A). For the luciferase reporter assay, a dual-luciferase construct was generated. Remarkably decreased luciferase activity was observed on pEZX-miR-201 co-transfecting with the pEZX-Luc-Caspase8ap2 3’UTR (Figure 4B; P < 0.01). These results showed that miR-210 could inhibit the activity of luciferase on the Caspase8ap2 3’UTR reporter gene cattier.
miR-210 mediated apoptosis and autophagy of H9C2 cells via targeted-regulating of Caspase8ap2
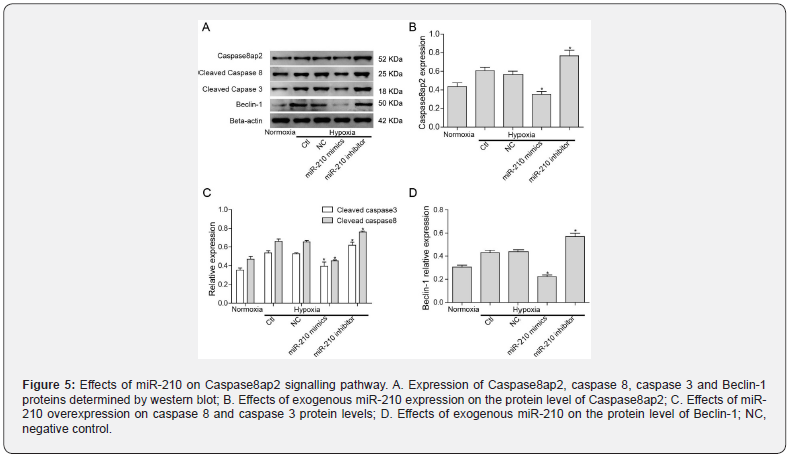
To further investigate the mechanism of miR-210 in regulating Caspase8ap2 and its associated signalling pathway, we determined the effects of exogenous miR-210 on the expression of Caspase8ap2 and downstream associated proteins by western blot (Figure 5A). Beclin-1 was upregulated in the hypoxia group compared with the normoxia group. Protein levels of Caspase8ap2, cleaved caspase 8 and cleaved caspase 3 were decreased in response to overexpressing miR-210 (Figure 5B and C). Beclin-1, which regulates both apoptosis and autophagy, was also downregulated in the miR-210 mimic group (Figure 5D). On the other hand, the expression of Caspase8ap2 and other proteins in response to silencing miR-210 were increased. Therefore, miR- 210 likely mediates apoptosis and autophagy in H9C2 cells via targeted-regulation of Caspase8ap2.
Discussion
Myocardial hypoxia is a negative prognostic marker associated with myocardial injury and a poor patient outcome. It is also a primary component of CHD microenvironment [13]. Recent work supports the idea that miRNAs are involved in the adaptive response to myocardial hypoxia in cardiovascular diseases. Liu et al. [14] suggested that miR-130a participated in hypoxia/reoxygenation (H/R) induced injuries by accelerating autophagy and relieving apoptosis in cardiomyocytes [14]. Moreover, the silencing of miR-122 alleviated cardiomyocyte H/R injury by promoting GATA-4 expression [15]. Li et al. [16] reported that miR-7a/b protected H9C2 cells from hypoxiainduced injury and promoted cardiac remodelling by decreasing fibrosis and apoptosis [16]. In particular, for the hypoxic response, all published literature agrees that miR-210 is a robust target of hypoxia-inducible factors. It is well known that the upregulation of miR-210 is a hypoxic signature in vivo [17]. Hence, miR-210- mediated myocardial hypoxia may represent an attractive target for therapeutic intervention in CHD. However, the mechanisms involved in miR-210 repair of myocardial hypoxia remains to be elucidated. Our study was performed to identify the role of miR- 210 in a hypoxic H9C2 model, which might contribute to the development of potential diagnostic and therapeutic approaches in CHD. First, we determined that a 48 h hypoxia treatment duration in H9C2 cardiomyocytes induced myocardial injury. Also, miR-210 significantly inhibited cell apoptosis and autophagy in hypoxic H9C2 cells. Since Caspase8ap2 was a putative target of miR-210, miR-210 likely mediated apoptosis and autophagy of H9C2 cells via suppressing Caspase8ap2. Taken together, the miR- 210/Caspase8ap2 pathway alleviated the effects of CoCl2 induced injury in H9C2 cells by regulating apoptosis and autophagy. There are few published articles on the effects of miR-210 on the other two apoptotic signaling pathways in cardiomyocytes. Besides of death receptor/ Caspase8 pathway, mitochondria/Caspase9 and ER stress/Caspase12 pathways are also major signaling pathways in apoptosis [18,19]. Further studies on the association between miR-210 and other potential target apoptotic genes in cardiomyocytes with hypoxia need to be conducted.
In the miRNA field, extensive evidence consistently points to a dominant role for miR-210 in the hypoxic stress response [20]. The induction of apoptosis in cells subjected to hypoxia has been of great importance to cell survival and tissue homeostasis. Numerous previous reports have supported an anti-apoptotic role of miR-210 during hypoxia. For example, miR-210 exhibited cytoprotective effects from apoptosis [10,21], while the downregulation of miR-210 induced apoptosis [22,23]. Similar results were verified in this study in which the overexpression of miR-210 inhibited apoptosis, whereas silencing miR-210 strikingly enhanced apoptosis in hypoxic H9C2 cells. Further, Caspase8ap2 has been predicted as a downstream target of miR- 210 [24]. Caspase8ap2 was initially identified as a member of the death signalling complex joining Fas and tumour necrosis factor-α (TNF-α) during apoptosis [12]. Its interaction with death effectors promotes the delivery of caspase-8 from the apoptosis pathway to activate the downstream caspase cascade. Because of its role in apoptosis, we validated Caspase8ap2 for its functional involvement in H9C2 apoptosis under hypoxic conditions using the dual-luciferase reporter assay. Western blot analysis demonstrated that miR-210 overexpression reduced the protein level of Caspase8ap2, cleaved caspase 8 and caspase 3 upon exposure to hypoxia, suggesting that miR-210 inhibited cardiomyocyte apoptosis by modulating the levels of Caspase8ap2.
The double-edged sword biological function of autophagy in myocardial hypoxia injury was demonstrated by recent studies. Moderate autophagy contributes to ATP generation and cellular homeostasis when cells are exposed to non-serious injury. However, uncontrolled excessive autophagy could be induced by serious or long-term injury. Excessive autophagy may contribute to autophagic cardiomyocyte death [25,26]. In our study, CoCl2 treatment for 48 h significantly inhibited cell survival. Hence, under this serious injury condition, we assumed that excessive autophagy was induced, and it may have contributed to cell death. Autophagy and apoptosis are considered to be highly interconnected through mechanisms that involve Beclin-1 [27]. Beclin-1 is necessary for autophagy to occur, it can direct autophagy-related proteins to phagocytes, and can regulate autophagy formation and maturation with other proteins. Although it is widely accepted that autophagy can promote survival in response to milder stress, excessive and/ or long-term autophagy can also cause cell death by promoting the excessive self-digestion of essential organelles and proteins. In the current study, both the upregulation of adapter protein p62 and downregulation of autophagy indicators LC3 II/I and Beclin-1 showed that miR-210 may protect H9C2 cells from excessive autophagy in response to hypoxia.
In conclusion, miR-210 collectively exerts anti-apoptosis and anti-autophagy effects, which alleviates myocardial injury in response to hypoxia. This study suggests that the miR-210/ Caspase8ap2 pathway is essential to protect cells from hypoxia. Future studies aimed at further characterizing the underlying mechanism of miR-210 in other apoptosis-associated pathways should be conducted.
Acknowledgements
We would like to give our gratitude sincere to the reviewers for their helpful comments on this paper. This study was supported by The National Key Clinical Speciality Construction Project of China (ZXA201101).
Conflict
There is no conflict of interest.
References
- Greenwood JP, Maredia N, Younger JF, Brown JM, Nixon J, et al. (2012) Cardiovascular magnetic resonance and single-photon emission computed tomography for diagnosis of coronary heart disease (CE-MARC): a prospective trial. Lancet 379(9814): 453-460.
- Rastam S, Al Ali R, Maziak W, Mzayek F, Fouad F M, et al. (2012) Explaining the increase in coronary heart disease mortality in Syria between 1996 and 2006. BMC Public Health 12(754): 754.
- Heran BS, Chen JM, Ebrahim S, Moxham T, Oldridge N, et al. (2011) Exercise-based cardiac rehabilitation for coronary heart disease. Cochrane Database Syst Rev 7: CD001800.
- Naoshad Muhammad, Sourav Bhattacharya, Robert Steele, Ratna B Ray (2016) Anti-miR-203 suppresses ER-positive breast cancer growth and stemness by targeting SOCS3. Oncotarget 7(36): 58595-58605.
- Yohei Shimono, Junko Mukohyama, Shun-Ichi Nakamura, Hironobu Minami (2015) MicroRNA Regulation of Human Breast Cancer Stem Cells. J Clin Med 5(1): 2.
- Feng J, Huang S, He M, Dai X, Li J, et al. (2014) Analysis on the association of single nucleotide polymorphism in the promoter region of pre-miR-320b-2 with coronary heart disease risk and factors influencing circulating microRNA-320b level. Zhonghua Yu Fang Yi Xue Za Zhi 48(10): 893-899.
- Miller CL, Haas U, Diaz R, Leeper NJ, Kundu RK, et al. (2014) Coronary heart disease-associated variation in TCF21 disrupts a miR-224 binding site and miRNA-mediated regulation. PLoS Genet 10(3): e1004263.
- Nabialek E, Wanha W, Kula D, Jadczyk T, Krajewska M, et al. (2013) Circulating microRNAs (miR-423-5p, miR-208a and miR-1) in acute myocardial infarction and stable coronary heart disease. Minerva Cardioangiol 61(6): 627-637.
- Huang X, Ding L, Bennewith KL, Tong RT, Welford SM, et al. (2009) Hypoxia-inducible mir-210 regulates normoxic gene expression involved in tumor initiation. Mol Cell 35(6): 856-867.
- Mutharasan RK, Nagpal V, Ichikawa Y, Ardehali H (2011) microRNA-210 is upregulated in hypoxic cardiomyocytes through Akt- and p53-dependent pathways and exerts cytoprotective effects. Am J Physiol Heart Circ Physiol 301(4): H1519-30.
- Hu S, Huang M, Jia F, Ghosh Z, Lijkwan MA, et al. (2010) MicroRNA-210 as a novel therapy for treatment of ischemic heart disease. Circulation 122(11 Suppl): S124-S131.
- Kim HW, Haider HK, Jiang S, Ashraf M (2009) Ischemic preconditioning augments survival of stem cells via miR-210 expression by targeting caspase-8-associated protein 2. J Biol Chem 284(48): 33161-33168.
- Kuo HF, Liu PL, Chong IW, Liu YP, Chen YH, et al. (2016) Pigment Epithelium-Derived Factor Mediates Autophagy and Apoptosis in Myocardial Hypoxia/Reoxygenation Injury. PLoS One 11(5): e0156059.
- Liu H, Huan L, Yin J, Qin M, Zhang Z, et al. (2017) Role of microRNA-130a in myocardial hypoxia/reoxygenation injury. Exp Ther Med 13(2): 759-765.
- Liang W, Guo J, Li J, Bai C, Dong Y (2016) Downregulation of miR-122 attenuates hypoxia/reoxygenation (H/R)-induced myocardial cell apoptosis by upregulating GATA-4. Biochem Biophys Res Commun 478(3): 1416-1422.
- Li R, Geng HH, Xiao J, Qin XT, Wang F, et al. (2016) miR-7a/b attenuates post-myocardial infarction remodeling and protects H9c2 cardiomyoblast against hypoxia-induced apoptosis involving Sp1 and PARP-1. Sci Rep 6: 29082.
- Huang X, Zuo J (2014) Emerging roles of miR-210 and other non-coding RNAs in the hypoxic response. Acta Biochim Biophys Sin (Shanghai) 46(3): 220-232.
- He B, Zhao Y, Xu L, Gao L, Su Y, et al. (2016) The nuclear melatonin receptor RORalpha is a novel endogenous defender against myocardial ischemia/reperfusion injury. J Pineal Res 60(3): 313-326.
- Pu J, Yuan A, Shan P, Gao E, Wang X, et al. (2013) Cardiomyocyte-expressed farnesoid-X-receptor is a novel apoptosis mediator and contributes to myocardial ischaemia/reperfusion injury. Eur Heart J 34(24): 1834-1845.
- Huang X, Le QT, Giaccia AJ (2010) MiR-210-micromanager of the hypoxia pathway. Trends Mol Med 16(5): 230-237.
- Nie Y, Han BM, Liu XB, Yang JJ, Wang F, et al. (2011) Identification of MicroRNAs involved in hypoxia- and serum deprivation-induced apoptosis in mesenchymal stem cells. Int J Biol Sci. 7(6): 762-768.
- Yang W, Sun T, Cao J, Liu F, Tian Y, et al. (2012) Downregulation of miR-210 expression inhibits proliferation, induces apoptosis, and enhances radio sensitivity in hypoxic human hepatoma cells in vitro. Exp Cell Res 318(8): 944-954.
- Gou D, Ramchandran R, Peng X, Yao L, Kang K, et al. (2012) miR-210 has an antiapoptotic effect in pulmonary artery smooth muscle cells during hypoxia. Am J Physiol Lung Cell Mol Physiol 303(8): L682-L691.
- Kim HW, Mallick F, Durrani S, Ashraf M, Jiang S, et al. (2012) Concomitant activation of miR-107/PDCD10 and hypoxamir-210/Casp8ap2 and their role in cytoprotection during ischemic preconditioning of stem cells. Antioxid Redox Signal 17(8): 1053-1065.
- Ma S, Wang Y, Chen Y, Cao F (2015) The role of the autophagy in myocardial ischemia/reperfusion injury. Biochimica et biophysica acta 1852(2): 271-276.
- Hamacher-Brady A, Brady NR, Gottlieb RA (2006) The interplay between pro-death and pro-survival signaling pathways in myocardial ischemia/reperfusion injury: apoptosis meets autophagy. Cardiovascular drugs and therapy 20(6): 445-462.
- Joubert PE, Werneke SW, de la Calle C, Guivel-Benhassine F, Giodini A, et al. (2012) Chikungunya virus-induced autophagy delays caspase-dependent cell death. J Exp Med 209(5): 1029-1047.






























