Augmentation of Zinc Concentration in Blood has a Favorable Effect on Cardiac Function Post-Myocardial Infarction
Fumitsugu Yoshikawa1, Kazuya Ooi2 and Ryuichi Aikawa2,3*
1 Kawasaki Hospital, Kobe city, Japan
2 Faculty of Pharmaceutical sciences, Suzuka University of Medical Science, Suzuka city, Japan
3 JCHO Sakuragaoka Hospital, Shizuoka city, Japan
Submission: April 15, 2020; Published: April 27, 2020
*Corresponding author: Ryuichi Aikawa, Faculty of Pharmaceutical sciences, Suzuka University of Medical Science, Suzuka city, Mie prefecture 516-8670, Japan
How to cite this article:Fumitsugu Y, Kazuya O, Ryuichi A. Augmentation of Zinc Concentration in Blood has a Favorable Effect on Cardiac Function Post- Myocardial Infarction. J Cardiol & Cardiovasc Ther. 2020; 16(2): 555933. DOI: 10.19080/JOCCT.2020.16.555933
Abstract
Background: We have recently reported that polaprezinc which is a zinc delivery has an anti-inflammatory effect and improves cardiac function after acute myocardial infarction (AMI). As a secondary analysis, the aim of the present study was to evaluate if zinc concentration in blood affects anti-inflammatory effect and cardiac function after AMI.
Methods: The primary study population included 50 patients with AMI. We equally divided the patients into two groups between the high group (H) and the low group (L) by blood concentration of zinc without relating to polaprezinc medication. The two groups were analyzed about cardiac function, cardiac enzymes, and the levels of the inflammation marker interleukin-6 (IL-6) as similar to the primary study.
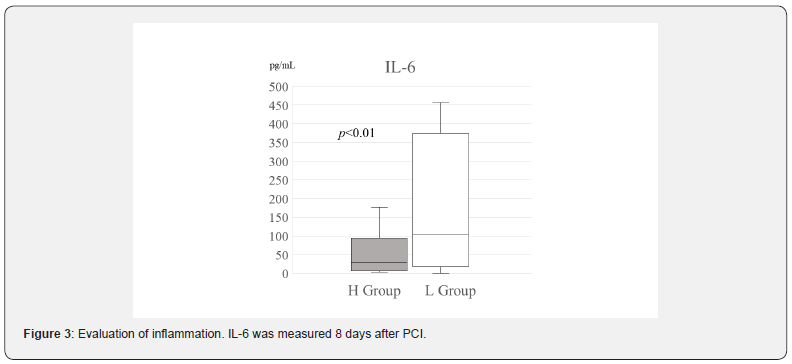
Results: The urine zinc levels of the H group were prominently higher than those of the L group at 8 days after PCI. The mean IL-6 level was strongly reduced in the H group (44.7(7.15-107.7) pg/mL vs. 130(19.6-384.25) pg/mL, respectively; p<0.05). As for the days of decline of both CRP and WBC, there were significant differences between the two groups (Figure 2). In addition, echocardiography indicated that the EF of the H group was clearly increased between day 3 and 9 months post-MI (54.5(50.5-59.75)% vs. 62(55-70)%, respectively; p<0.01).
Conclusions: The present study suggests that high concentration of zinc has an anti-inflammatory effect and improves cardiac function after AMI.
Keywords: Zinc; Acute myocardial infarction; Inflammation; Cardiac function
Abbreviations: AMI: Acute Myocardial Infarction; PCI: Percutaneous Coronary Intervention; IL-6: Interleukin-6; CPK: Creatine Phosphokinase; EF: Ejection Fraction; CRP: C-Reactive Protein; LVDd: Left Ventricular End-Diastolic Dimension; LVDs: Left Ventricular End-Systolic Dimension; BMS: Bare Metal Stent; DES: Drug Eluting Stent; BMI: Body Mass Index; DM: Diabetes Mellitus; WBC: White Blood Cell; ZIP: Zrt, Irt-Like Protein; ZnT: Zinc Transporter
Introduction
Zinc is one of the most abundant trace elements in the human body [1,2]. It is involved in antioxidant activity, immunity and inflammation [3-5]. Acute myocardial infarction (AMI) is a major cause of death worldwide [6,7]. It has recently been reported that zinc supplementation attenuates cardiac remodeling after experimental myocardial infarction in rats through implementing [8]. Several studies have indicated that intracellular zinc homeostasis is tightly regulated by two known classes of zinc transporters [9,10]. One of the transporters, Zrt, Irt-like protein (ZIP), has functions in the uptake of zinc into the cytoplasm of cell from either the extracellular space or from intracellular organelles [10]. The other one, Zinc transporter (ZnT), moves zinc from the cytoplasm to the outside of the cell or into the lumen of intracellular organelles [11]. It has been reported that the transporters are markedly abundant in human heart muscle tissues [12], and intracellular zinc level is decreased during reperfusion injury in cardiomyocytes due to lowered expression of ZIPs [13]. It is highly possible that intracellular zinc level of cardiomyocytes should be decreased in infarcted hearts. Therefore, supplementation of zinc may be useful for the patients with AMI. We thus performed a study using a zinc derivative, polaprezinc (Zeria Pharmaceutical Co., Ltd., Tokyo, Japan) for the patients with AMI previously. The patient group taking polaprezinc was significantly increased zinc concentration in both blood and urine, and reduced the level of inflammation marker IL-6 compared to the other control group without polaprezinc. In addition, the ejection fraction (EF) of the polaprezinc group was prominently augmented compared to the non-polaprezinc group in echocardiography. Subsequently, we demonstrated that polaprezinc had an anti-inflammatory effect and improves cardiac function after AMI [14]. However, there were some patients showing high concentration level of zinc in blood and urine without polaprezinc medication in the primary study indeed. Particularly, one patient was consuming daily a large amount of green tea containing high levels of zinc [14]. Although this patient was in the control group, the zinc level in his peripheral blood and urine were much higher than the average level in the polaprezinc group [14]. Conversely, there were some patients showing the low level of zinc in spite of taking polaprezinc [14].
Accordingly, we performed a secondary analysis of our previous study. The aim of the present study was to evaluate if zinc concentration in blood affects anti-inflammatory and cardiac function post-AMI by dividing into two groups, high concentration of zinc group and low concentration of zinc group.
Methods
The primary study population included 50 patients with initial ST elevation myocardial infarction between September 2011 and July 2014. The patients underwent PCI successfully within 12 h from the onset of AMI. After PCI, they were randomly administered 75 mg of polaprezinc twice a day for 9 months. Peripheral blood and urinary samples were collected in a specific time to analyze the zinc concentration, cardiac enzymes, and the level of inflammation markers (including IL-6) at 8 days after PCI. All patients administered statin from 8 days after PCI. To evaluate the cardiac function in terms of the EF, LVDd, and LVDs, echocardiography was performed upon admission to the hospital and at 9 months post-AMI. The LVDd and LVDs were determined according to the guidelines of the American Society of Echocardiography. The EF was estimated using the modified Simpson method.
In the present study, 4 patients were excluded from the primary study because they had been not analyzed the zinc concentration in blood. We thus enrolled 46 eligible patients, and they were equally divided into two groups between high concentration of zinc group (H group, n=23) and low concentration of zinc group (L group, n=23) according to the median serum zinc concentration of 93 mg/dl as a standard. Here we analyzed two groups with the difference of zinc concentration in blood on anti-inflammatory effect and cardiac function after AMI.
Percutaneous coronary interventions
All patients received a single dose of 300 mg clopidogrel and 200 mg aspirin pre-PCI. Heparin was also administered in the catheterization laboratory. After PCI, patients continued to take 100 mg aspirin and 75 mg clopidogrel daily until the follow-up study. The PCI was performed according to the transfemoral, transradial, and transbrachial approaches, and the puncture was performed using 6 F or 7 F sheaths, as shown in Table 1. If necessary, an aspirator was used during PCI, such as TVAC (Nipro®, n=17) and Thrombuster (Kaneka®, n=21). The bare metal stents (BMS) used included Driver (Medtronic®, n=2), Liberte (Boston Scientific®, n=1), S-Stent (St. Jude Medical®, n=2), and Integrity (Medtronic®, n=7). The drug eluting stents (DES) used included Nobori (Terumo®, n=15), Endeavor (Medtronic®, n=4), Resolute (Medtronic®, n=3), Xience (Abbott®, n=6), and Promus (Boston Scientific®, n=6). Significant differences were not detected in the number of responsible branches for AMI (Table 1).

Statistical Analysis
Continuous data are presented as the median with interquartile range, and categorical data are presented as the number (percentage). The present study had a small sample size, and thus non-parametric statistical analysis methods were employed. Differences between continuous variables were analyzed using Mann-Whitney U-test. Categorical data were compared using Fisher’s exact test. Mann-Whitney U-test was used to compare the EF, LVDd, and LVDs detected by echocardiography during the follow-up. A p value of less than 0.05 was considered to indicate a statistically significant difference. All statistical analyses were performed with EZR software (Saitama Medical Center, Jichi Medical University, Saitama, Japan) [15].
Results
In more detail, there contained 16 people who took polaprezinc and 7 people who did not take polaprezinc in the H group (n=23). On the other hand, there contained 8 people who took polaprezinc and 15 people who did not take polaprezinc in the L group (n=23). There was some statistical significance in the clinical characteristics at the baseline which was detected between the H and the L groups (Table 1). A number of young patients (p<0.01), male rate (p<0.05) and BMI of patients (p<0.01) were observed in the H group compared to the L group. It is established that CPK is used as a marker for myocardial infarct size [16-18]. In the primary study, there was a problem for data analysis because there had a significance in the maximal (MAX) CPK between two groups [16]. In that case, we needed to rectify other biochemical markers by the significant difference of MAX CPK [16]. In the present study, there was not difference between the maximal CPK in the H group and in the L group. It suggests that there had the same level of cardiac muscle injury after myocardial infarction, and that we did not need to correct the data between two groups.
By the zinc concentration in blood, the patients were clearly divided at the 93μg/dL into the H group and the L group (105(96- 114) vs. 77(64-84) μg/dL, respectively; p<0.01) at 8 days after AMI (Figure 1A). The urine zinc level of the H group was significantly higher in comparison to that of the L group (945(738-1780) vs. 653(474-1030) μg/L, respectively; p<0.01) (Figure 1B).
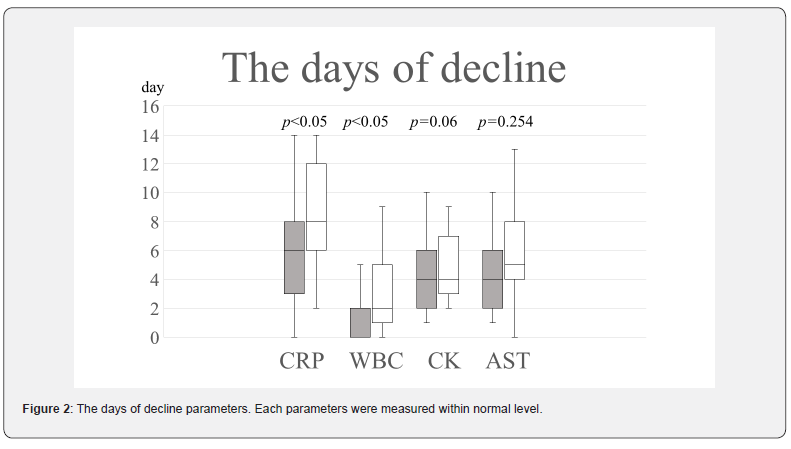
Recent studies have shown that inflammatory markers, such as CRP and IL-6, are more important than maximal CPK as prognostic factors of AMI [19,20]. Thus, IL-6 level, CRP and WBC were also analyzed. We measured the days of decline CRP, WBC, CK and AST. As for AST and CK, there were no significant differences between the two groups (Figure 2). However, as for CRP and WBC, there were significant differences between the two groups (Figure 2). An important inflammatory cytokine, IL-6 was also measured as shown previously. IL-6 was significantly lower in the H group than that in the L group, as shown in Figure 3 (28.9(6.9-94.0) pg/mL vs. 157(22.2-379) pg/mL, respectively; p<0.05). About the cardiac function, there had no difference in LVDd (Figure 4A), LVDs (Figure 4B) and EF (Figure 4C) in the L group between day 3and 9 months post-AMI in echocardiography. By contrast, echocardiography indicated that the EF of the H group was significantly increased between day 3and 9 months post-MI (55(51.5-60.3) % vs. 62(55-70) %, respectively; p<0.01) (Figure 4C). By 9 months post-AMI, 2 patients were lost to follow-up in the H group and 1 patient was lost to follow-up in the L group, since these 3 patients were followed up by other hospitals after moving.


Discussion
In the original study, there were differences of the MAX CPK between in the control group and in the polaprezinc group [14]. In that case, we needed to rectify the IL-6 value by the significant difference of the MAX CPK. Because in the present study there was no difference between the MAX CPK in the H group and in the L group, we did not need to correct the biochemical markers.The days of decline CRP and WBC in the H group was less than those of the L group (Figure 2). Also, the L-6 value was significantly lower in the H group than that in the L group, as shown in Figure 3. These results indicate that an inflammatory reaction was significantly suppressed in the H group. It is well known that statin drugs can reduce inflammation in AMI [21]. However, statin is not involved in this study about the results entirely, because statin medication was started 8 days after AMI in this study until taking blood for examination of IL-6. Moreover, the present study suggests that the serum high level of zinc significantly reduced directly the IL-6 value, not limited to medication of polaprezinc.
In the primary study, the male rate was not significant between two groups (polaprezinc group and control group). However, the male rate in the H group was higher than in the L group in this secondary study. Ministry of Health, Labor and Welfare in Japan recommended intake of 10 mg/day and 8 mg/day for adult males and females, respectively [22]. It seems to be different in the metabolism of zinc between men and women. The tendency of gender difference in the level of zinc may be preserved in the patients with AMI. Moreover, compared to the patients of the H group, the age and the BMI value of patients were lower in the L group. Although there was a difference in the BMI of the AMI patients between two groups in our previous study, there was no difference in the age between them [14]. The recent studies have showed that elderlies are prone to zinc deficiency [23,24]. Possibly, young people may be able to retain the zinc in the body, and the concentration of zinc becomes lower with the advance of age. In terms of BMI, it already reported that Zn levels in obese patients were significantly lower than in healthy controls [25]. Our result was different from the previous study of Italy [25]. In general, adult obesity is highly connected to progression of atherosclerosis and epidemiological evidence suggests that overweight and obesity have been associated with acute myocardial infarction [26,27]. In this study, the patients with AMI contained more young people (under 65) in the H group compared to the L group (52.1% vs 34.7%). It is conceivable that the age may be involved in the relationship between the BMI and Zinc level in the H group.
In addition, it has recently been focused that AMI is correlation with inflammation [28]. Our previous first study demonstrated that IL-6, a critical inflammation biomarker was lowered in the group which took polaprezinc [14]. However, this mechanism is still not clarified. In this analysis, the high group of zinc concentration had an effect on suppressing not only IL-6 (Figure 3) but also WBC and CRP (Figure 2). IL-6 is made mainly by one type of white blood cell, the macrophage [29]. Furthermore, it is known that several cytokines increased in response to the breakdown of myocardial tissue promote the production of CRP [30]. Therefore, this study suggests that zinc may inhibit inflammatory reaction by suppression of WBC function in the beginning in AMI patients. Further studies are required to clarify the suppressive effect of zinc on IL-6 production.
The present study showed that the high serum level of zinc significantly improved the EF in post-AMI patients (Figure 4C). Therefore, we assume that zinc may be involved in the process to repair cardiac muscle after AMI. Recently, Willson et al. [31] also stated that zinc deficiency plays a major causal role for apoptosis in the pathology of in the skin [31]. Also Lizzi et al. [32] has reported that the potential role of autophagy as a mediator of the protective effects of zinc [32]. It suggests that zinc may protect a cardiomyocyte by regulating cell death after myocardial infarction.
Study Limitations
The present study has several limitations. First, the sample size was fairly small. The limitations of this study also include the absence of a multicenter trial and significant differences in the percentage of BMI and age between the two groups. In addition, the study excluded patients with severe MI or inflammation disease. Furthermore, four patients were lost to follow-up during the study. Finally, the PCI procedures were performed by multiple doctors.
Conclusion
Preserving serum high concentration of zinc improved cardiac function post-AMI. This effect may be due to anti-inflammatory processes through the suppression of IL-6. In particularly, elderly patients has a tendency of zinc deficiency, they should be provided zinc ingredient and supplement.
Acknowledgments
The authors would like to thank Dr. Yokoi, Dr. Kondo, Dr. Tamura, Dr. Watanabe, Dr. Natsuaki, and Dr. Morishita for performing PCI and data collection.
References
- Hojyo S, Fukada T (2016) Zinc transporters and signaling in physiology and pathogenesis. Arch Biochem Biophys 611:43-50.
- Maret W (2013) Zinc biochemistry: from a single zinc enzyme to a key element of life. Adv Nutr 4(1): 82-91.
- Brown KH, Peerson JM, Rivera J, Allen LH (2002) Effect of supplemental zinc on the growth and serum zinc concentrations of prepubertal children: a meta-analysis of randomized controlled trials. Am J Clin Nutr. 75(6): 1062-1071.
- Powell SR (2000) The antioxidant properties of zinc. J Nutr 130(5S Suppl): 1447S-1454S.
- Gammoh NZ, Rink L (2017) Zinc in Infection and Inflammation. Nutrients 9(6): 624.
- Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJ (2006) Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet 367(9524): 1747-1757.
- Tomoike H, Yokoyama H, Sumita Y, Hanai S, Kada A, et al. (2015) Nationwide distribution of cardiovascular practice in Japan - results of Japanese circulation society 2010 annual survey. Circ J 79(5): 1058-1067.
- Gonçalves AF, Polegato BF, Fernandes AA, Ishikawa LL, Okoshi K, et al. (2018) Zinc Supplementation Attenuates Cardiac Remodeling After Experimental Myocardial Infarction. Cell Physiol Biochem 50(1): 353-362.
- Fukada T, Kambe T (2011) Molecular and genetic features of zinc transporters in physiology and pathogenesis. Metallomics 3(7): 662-674.
- Kambe T (2011) An overview of a wide range of functions of ZnT and Zip zinc transporters in the secretory pathway. Biosci Biotechnol Biochem 75(6): 1036-1043.
- Choi S, Bird AJ (2014) Zinc'ing sensibly: controlling zinc homeostasis at the transcriptional level. Metallomics 6(7): 1198-1215.
- Choi S, Liu X, Pan Z (2018) Zinc deficiency and cellular oxidative stress: prognostic implications in cardiovascular diseases. Acta Pharmacol Sin 39(7): 1120-1132.
- Bodiga VL, Thokala S, Kovur SM, Bodiga S (2017) Zinc Dyshomeostasis in Cardiomyocytes after Acute Hypoxia/Reoxygenation. Biol Trace Elem Res 179(1): 117-129.
- Yoshikawa F, Nakajima T, Hanada M, Hirata K, Masuyama T, et al. (2019) Beneficial effect of polaprezinc on cardiac function post-myocardial infarction: A prospective and randomized clinical trial. Medicine (Baltimore) 98(10): e14637.
- Kanda Y (2013) Investigation of the freely available easy-to-use software “EZR” (Easy R) for medical statistics. Bone Marrow Transplant 48(3): 452-458.
- Sobel BE, Bresnahan GF, Shell WE, Yoder RD (1972) Estimation of infarct size in man and its relation to prognosis. Circulation 46(4): 640-648.
- Norris RM, Whitlock RM, Barrat-Boyes C, Small CW (1975) Clinical measurement of myocardial infarct size. Modification of a method for the estimation of total creatine phosphokinase release after myocardial infarction. Circulation 51(4): 614-620.
- Halkin A, Stone GW, Grines CL, Cox DA, Rutherford BD, et al. (2006) Prognostic implications of creatine kinase elevation after primary percutaneous coronary intervention for acute myocardial infarction. J Am Coll Cardiol 47(5): 951-961.
- Tan J, Hua Q, Li J, Fan Z (2009) Prognostic value of interleukin-6 during a 3-year follow-up in patients with acute ST-segment elevation myocardial infarction. Heart Vessels 24(5): 329-334.
- Fisman EZ, Benderly M, Esper RJ, Behar S, Boyko V, et al. (2006) Interleukin-6 and the risk of future cardiovascular events in patients with angina pectoris and/or healed myocardial infarction. Am J Cardiol 98(1): 14-18.
- Suna S, Sakata Y, Sato H (2009) Inflammation and risk management in patients with coronary heart disease. J Jpn Coron Assoc 15: 171-177.
- Komai M (2015) Present situation of dietary zinc ingestion in Japanese population and short comments for dietary reference intake. Nutr Ther Zinc 6: 4-11 (in Japanese).
- Yasuda H, Tsutsui T (2016) Infants and elderlies are susceptible to zinc deficiency. Sci Rep 6: 21850.
- Kurasawa R, Kubori S (2006) Zinc Deficiency and its clinical features in the cases found in Kitamimaki, a rural area in Japan. Biomed Res Trace Elements 17: 91.
- Di Martino G, Matera MG, De Martino B, Vacca C, Di Martino S, et al. (1993) Relationship between zinc and obesity. J Med 24(2-3): 177-183.
- Aprahamian TR, Sam F (2011) Adiponectin in cardiovascular inflammation and obesity. J Inflam 376909.
- Zhu J, Su X, Li G, Chen J, Tang B, et al. (2014) The incidence of acute myocardial infarction in relation to overweight and obesity: a meta-analysis. Arch Med Sci 10(5): 855-862.
- Prabhu SD, Frangogiannis NG (2016) The Biological Basis for Cardiac Repair After Myocardial Infarction: From Inflammation to Fibrosis. Circ Res 119(1): 91-112.
- Kishimoto T (2010) IL-6: from its discovery to clinical applications. Int Immunol 22(5): 347-352.
- Nian M, Lee P, Khaper N, Liu P (2004) Inflammatory cytokines and postmyocardial infarction remodeling. Circ Res 94(12): 1543-1553.
- Wilson D, Varigos G, Ackland ML (2006) Apoptosis may underlie the pathology of zinc-deficient skin. Immunol Cell Biol 84(1): 28-37.
- Liuzzi JP, Guo L, Yoo C, Stewart TS (2014) Zinc and autophagy. Biometals 27(6): 1087-1096.






























