Catheter Ablation of Premature Ventricular Complexes Exclusively by a Pace-Mapping Software Technique: Feasibility of a Registry Study
Fabricio Sarmento Vassallo1,2*, Eduardo Serpa1,2, Lucas Luis Meigre1, Edevaldo Silva3, Rafael Zeni3, Christiano Cunha1,2, Hermes Carloni2, Aloyr Simoes Jr2, Karla Meira1, Flávia Pezzin1, Walter Duarte Batista Jr2, Alberto de Paula Nogueira Jr2, Dalton Hespanhol2 and Carlos Lovatto2
1 Institute of Cardiology of Espirito Santo, Brazil
2Santa Rita de Cassia Hospital, Brazil
3 Abbott Brazil
Submission: April 15, 2020; Published: April 27, 2020
*Corresponding author: Fabricio Sarmento Vassallo, Coordinator of Electrophysiology of Institute of Cardiology Espirito Santo and Santa Rita de Cassia Hospital, Brazil
How to cite this article: Fabricio S V, Eduardo S, Lucas L M, Edevaldo S, Rafael Z, et al. Catheter Ablation of Premature Ventricular Complexes Exclusively by a Pace-Mapping Software Technique: Feasibility of a Registry Study. J Cardiol & Cardiovasc Ther. 2020; 16(2): 555932. DOI 10.19080/JOCCT.2020.16.555932
Abstract
Introduction/Objective: Ablation of premature ventricular complexes a challenge in routine electrophysiology because results, depend on factors such as location, tools and technologies used. Aim of study was analyze exclusive use of pace-mapping technique with module of Score Map Software of Ensite Velocity/Precision and compare with success procedure, 7- and 30-days post-ablation.
Methods/Results: Retrospectively analyzed 50 procedures in 47 symptomatic patients between January 2018 and February 2020. Ablation with irrigated tip catheter, power 40Watts, pump flow 30ml/min. Mapping with Duodecapolar in 40(80%) and HDGrid catheter in 10(20%). Female was 25 (53.19%), mean age 53.5years (19-78), hypertension 27 (57.45%), 7 (14.91%) coronary disease, 4 (8.51%) myocarditis, 2 (4.26%) Diabetes and 2 (4.26%) patients with an implanted defibrillator. Mean ejection fraction 63.17%. Average radiofrequency time was 580.58 seconds and mean XRay time 7.74min. Median SM was 93.92% and only 8 (16%) ablations were considered unsuccessful due to maintenance of extrasystoles or do not show a marked reduction on PVC at the end of the procedure. At 7- and 30-days follow up 9 (18%) and 7 (14%) patients had unsuccessful ablation. All patients had a Score <95%, underwent ablation with conscious sedation, majority female, most PVCs located in left ventricle and had more extensive ablation.
Conclusion: Score Map is useful as exclusive technique for ablation of premature ventricular complexes with very good acute success and low complications. Score Map more than 95% had high success. Failure is related to conscious sedation, female gender, left ventricle ablation and longer radiofrequency time.
Keywords: Premature ventricular beats; Catheter ablation; Pace-mapping; Electroanatomic mapping and activation map
Introduction
Radiofrequency (RF) catheter ablation (CA) is an effective treatment for symptomatic, idiopathic premature ventricular complexes (PVCs) [1-4]. The mechanisms of these arrhythmias tend to be triggered activity or abnormal automaticity, as opposed to reentry which more commonly underlies sustained ventricular arrhythmias most common in the sets of patients with structural heart disease [5-7]. Nowadays, in the era of electroanatomic systems supporting the ablation of PVCs, activation mapping is the preferred strategy to localize the site of origin and guide successful application of RF [8-11]. However, detailed activation mapping requires sufficient intraprocedural quantity of PVCs, which is a very common issue since anesthetic medications are used during the procedure leading in a reduction and occasionally absent of PVCs despite the utilization of all available stimulation protocols and medications to sensitize the foci of the arrhythmia. Pace‐mapping has been since long time ago utilized to guide ablation of idiopathic PVCs [12-14], but there is a lack of clinical data on the use of pace‐mapping commercials software’s available in the electroanatomic mappings as an exclusive strategy for ablation of infrequent PVCs [15].
Objective
The aim of this study was to investigate the acute outcomes of CA in patients with infrequent intraprocedural PVCs guided exclusively by a commercial pace‐mapping software and its feasibility as a routine approach.
Methods
This is an observational, retrospective study, that utilized patients submitted to catheter ablation for treatment and elimination of symptomatic ventricular ectopic beats exclusively with the support of a pace mapping score software presented at the EnSite Velocity/Precision Electroanatomic Mapping System named Score Map (St Jude Medical / Abbott USA).
A) Study population
We retrospectively analyzed 50 procedures performed in 47 patients with symptomatic [16] premature ventricular complexes between January 2018 and February 2020, with a rate of 1.06 procedure per patients. Most common symptoms were palpitations, fatigue, shortness of breath and new onset of functional limitation. Female gender in 25 (53.19%) patients, mean age of 53.5years (19-78), hypertension in 27 (57.45%), 7 (14.91%) coronary disease and 2 (4.26%) with Diabetes. Majority of cases, 40 (85.11%) was idiopathic, 5 (10.63%) with previous myocarditis and 2 (4.26%) with adult corrected congenital cardiopathy. Two patients (4.26%) had and implanted cardio defibrillator, one for secondary prevention after a delayed correction for interatrial communication and one after a myocarditis and sustained ventricular tachycardia with syncope. Mean ejection fraction 63.17% (47.35-77%) with mean end diastolic diameter of 49.56 (42-62) mm. Previous medication before ablation were beta-blockers in 31 (65.96%), amiodarone in 14 (29.79%) and sotalol in 13 (27.66%) patients. The mean time between catheter ablation and the documentation of the symptoms were 10.4 (3-21) months. Clinical features are described in Table 1.
B) Ensite Velocity Automap Module
The EnSite™ AutoMap Module is a software entitlement feature within the EnSite™ Cardiac Mapping System. AutoMap Module automates the point collection process, based on the user-defined settings. As the mapping catheter is moved, points are collected when the user-defined settings are met. Score Map is a map type that plots the surface ECG morphology match score associated with each mapping point collected compared to the originally collected template beat surface lead morphology. The physician may want to use this feature during pace mapping using an adjustable percentage morphology match score in the area of interest. The technician and/or physician may also sort the points list by score, from highest to lowest score, comparing to surface ECG morphology beat. This may be useful to determine which surface leads are different from the template beat. Score Map (SM) can also be used during automated mapping to allow users to automatically collect only those points with morphology match scores that are XX% similar or higher compared to the template beat and automatically reject those points that are not XX% similar or higher compared to the original template beat. When Start AutoMap is activated, the software automatically records the data from 10seconds before the EnSite™ AutoMap feature was activated and continues recording as long as the EnSite™ Auto Map feature is active [17].
C) Pace-Mapping Technique and Catheter Ablation
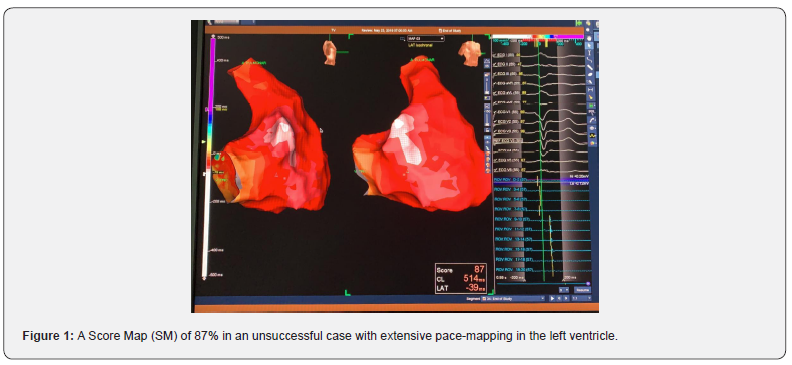
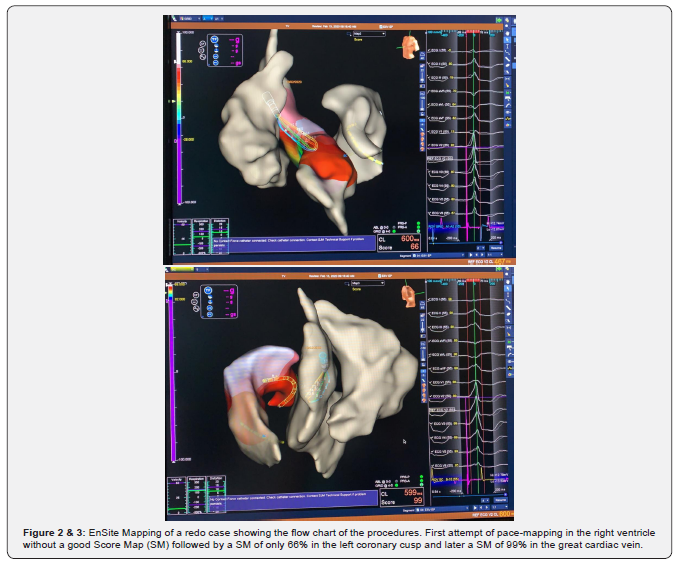
Procedure was done in a fasting state. Previous to the start of the anesthesia a 12-lead ECG on the electrophysiologic (EP) recording system and also on the EnSite Velocity 5.0 with a variable time recording to document at least several clinical PVCs morphologies for proceeding with ablation. After that and at the discretion of the operator the anesthesia and electrophysiologic study was started. Minimum Score Map points accepted to be collected in the achievement and construction of the morphology of the chamber was 90% in all cases except in 6 (12%), that a best SM reached was between 84 and 88% (Figure 1) and in these cases our option was to move to another ventricular chamber. If this conduct does not achieve a better SM we started a new map in the coronary sinus and their branches (Figures 2 & 3). The ablation procedure was done with Flexability and TactiCath SE catheters in 48 and 2 cases respectively, with a power of 40 Watts and a pump flow of 30ml/min and in the cases of ablation in the coronary sinus system the power was titrated to 15 to 20 Watts and flow pump maintained in the same flux. Mapping catheters utilized was the Livewire duodecapolar 2-2-2 catheter in 40 (80%) and Advisory HDGrid catheter in 10(20%) patients. Reference of the 3D mapping system was an Irvine decapolar catheter inserted in the coronary sinus that was attached in the femoral access (all catheters in the St Jude Medical / Abbott – USA portfolio).

D) Informed Consent and Ethical Considerations
All patients signed the informed consent form according to the standards of the institution which follows national and international standards [18,19]. The study was approved by the Research Ethics Committee of the Institution.
E) Post-Ablation Follow-UP
Patients was discharged from the Hospital on the day after of the catheter ablation and received orientations and scheduled two appointments with 7 and 30 days after the procedure. On theses clinicals examinations a clinical questionnaire and an electrocardiogram with a 5 to 10 minutes monitoring to seek for PVCS were done in all cases.
F) Statistical Analysis
All tests were performed using BioStat statistical software (by AnalystSoft Walnut, CA). Continuous variables were expressed as mean ± SD or (Min / Max) or as median and interquartile range (IQR). P value was considered significant if < 0.05 by the chisquare test.
Results
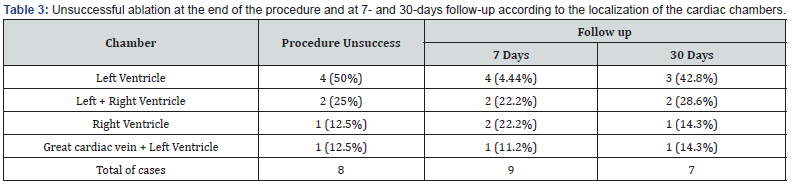
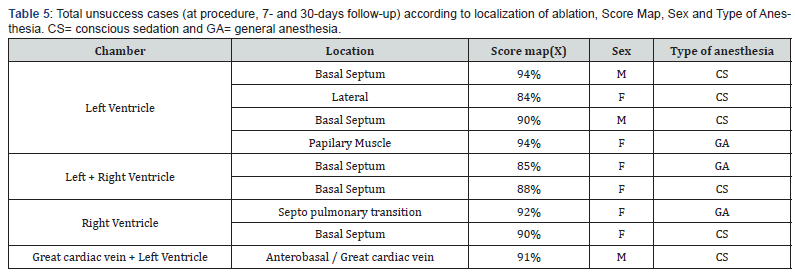
Forty-seven symptomatic patients underwent 50 procedures between January 2018 and February 2020. Median SM was 93.92 (84-100) %. Average Radiofrequency (RF) time was 580.58 (176- 1356) seconds with shorter times for success and longer for unsuccessful cases, with 356.12 seconds and 829.51 seconds, respectively. Beside this difference in RF times there were a nonsignificant P of 0.4457. Mean X Ray time 7.74 (1.9-33) min (Table 2). In 16 (32%) patients during the RF application we observed an accelerated ventricular rhythm with a high score of similarity with the clinical PVC. The most common localization of the PVCs was at the right ventricle in 21 (42%) ablations, followed by left ventricle in 12 (24%), left coronary cusp in 5 (10%) and right coronary cusp, anterolateral cardiac vein in 1 (2%) case each. The last ablation sites were done in two chambers when the PVCs was not eliminated targeting only one place, being great cardiac vein plus left ventricle (LV) in 3 (6%), right ventricle (RV) plus right coronary cusp in 3 (6%), both ventricle in 3 (6%), and right coronary plus non-coronary cusp in 1 (2%) case. Only 8 (16%) ablations were considered unsuccessful due to a maintenance of the same PVCs density duration ablation or their return after the recovery of the anesthetic status. In these cases, most unsuccessful sites were in the LV in 4 (50%) ablations (Figures 4 & 5), LV/RV in 2 (25%) and 1 (12.5%) in LV plus great cardiac vein and 1 (12.5%) in RV locations. At 7days of follow-up a total of 9 (18%) cases of unsuccessful ablations were seen. The same patients with intraprocedural failure added by one patient with recurrence of the clinical PVCs in a RV ablation. When patients were assessed at 30-days a mild reduction on the maintenance of PVCs was observed, all in the same cases of failure at the index procedure less one, so seven cases (14%) were then unsuccessful at one month after catheter ablation. This 1 case with ablation on the septal region of booth ventricles that initially was considered unsuccessful at the end of ablation, do not show PVCs at this time of follow up (Table 3). Making a secondary analyze by taking the localization of the chamber receiving the catheter ablation and its correlation with success and unsuccess, when ablation was done in LV associated or not with other chambers, RV or coronary sinus, this, was a marker of unsuccess with a significant P of 0.00208. But this is not true when we compare ablation only at LV with other chambers as a marker of an unsuccess procedure, showing a P of 0.08 (Table 4). In unsuccessful cases, all patients had a SM below or equal 94%, underwent ablation with conscious sedation, majority were female, PVCs ablated at the basal septum of the left and left/ right ventricles and had longer times of radiofrequency. When we did an analysis of the type of anesthetic approach as a marker of intraprocedure success we saw the presence of conscious sedation in 6 (75%) of 8 unsuccessful procedures and 6 (14.29%) in 42 successful with a highly significant P value of 0.002551 (Table 5). The rate of complications was low with only one (2%) pericardial effusion managed with a percutaneous pericardial drainage, one pseudoaneurysm (2%) resolved with local doppler compression and 4 (8%) of local bruises/hematomas.

Discussion
As the mechanism of idiopathic premature ventricular complexes is mostly triggered activity or abnormal automaticity, activation mapping is the preferred strategy to localize the site of origin and guide successful ablation, however, fluctuations in PVC burden during catheter ablation procedures is a common finding with multiple explanations that’s includes circadian variability [20], emotional status and effects of the type of anesthesia and the anesthetic drugs used. On the well demonstrated paper of Shirai et all. a solely strategy of the mapping and ablation outcomes for patients in whom intraprocedural PVCs after conscientious sedation or general anesthesia is so low or absent that this impacts in the adequate construction of a trustful activation mapping. This exclusive approach with extensive pace‐mapping guided module with automated morphology matching capability with posterior ablation showed a 97% of pace-mapping matches score and achieved a reasonably high clinical success rate of 79% at 9 months of follow-up. Comparing to our results we saw a less efficient SM score of 93.92% but a slightly higher success of 86% at 30 days of follow-up. On them methodology the activation mapping was also used if the targeted PVC was observed during the procedure and in our cases, we only used the pace-mapping with SM. In another previous study of Gerstenfeld et al. [14] showed that pace‐mapping with the utilization of a 12‐lead ECG quantified waveform mean absolute deviation score was sensitive enough to avoid ablation at poor sites and on their manuscript septal locations was associated with less successful ablations. Two years later Azegami et al. [21] reported that pace‐mapping, based on a 24‐point matched score, provided similar localizing information as in activation mapping using the Carto Mapping System. In this study, although multiple best pace‐mapping sites were separated by a greatest distance of 18±5mm, the probability of obtaining an exact perfect pace mapping match was higher within 5mm of the site of earliest activation. In 2008 Bojun et al. [22], also with Carto system, utilizing a 12‐point scoring system, reported the spatial resolution of a good pace‐map in the RVOT was 1.8 cm2, which was inferior to the spatial resolution of activation mapping as assessed by isochronal activation, but still able to reliably identify the site of origin in 82% of patients. In addition, in this particularly study, mapping of the LVOT was not performed, allowing for the possibility of earlier activation directly across from the septal RVOT. These two studies showed that using of pace mapping score techniques comparable with activation map are feasible for performing PVCs ablations. Another point that have to be discussed is the challenge of pace mapping during the procedure. There are several of those that can limit success even in labs with high volume and experience We will address some here; A) high current outputs to achieve capture is occasionally required within certain fibrous regions such as the RV/pulmonary transition and the coronary cusps. It can result in a large virtual electrode effect with the possibility deformation of the paced electrocardiogram and simultaneous capture of remote areas potentially decreasing the precision of the SM. So, it is very important to gradually decrease the output in an effort to capture individual fibers that can result in a reproducibly longer stimulus to QRS interval and a change in QRS morphology. Pace‐mapping just above the capture threshold and with a coupling interval similar to the targeted PVC was performed to identify the putative site of origin. B) like in previous reports [23,24], beside a good correlation, sites with the earliest activation time can be spatially distant from sites with better pace mapping due the presence of abnormal insulated fibers of myocardium on epicardial aspect of the fibrous walls of the valvar sinuses. Another site with these characteristics is the papillary muscles. C) for a reasonable pace mapping matched technique, it is imperative that the basal PVC used in the system is achieved with the ECG acquired in the EP Lab. This is our routine on cases submitted to PVC ablation because if there is no PVCs during the monitoring the patients there are higher chances to put the ECG leads on different positions and the success of the ablation will be certainly limited because small changes in electrode location can lead to important changes in morphology [25]. D) in some cases, sites of PVC origin can be intramural with distinct exit sites depending on the myofiber or myolaminar orientation and when those occurs, RF delivery at best ablation site can alter the PVC morphology reflecting a change in exit site without eliminating then. In a recent study, Li et al. [26], demonstrated that significant differences in spatial resolution of pace mapping existed within the heart. With this knowledge, when pace mapping matched score is the option to guide ablation an adequate number of points around the area of interest is required to obtain higher success rates. So, in cases of an exclusive pace mapping score approach, even with a high SM rate it may be reasonable in comparison to activation map, to make more extensive pace-mapping with more RF ablation lesions since the absence or even a low burden of PVCs limits the success endpoint. This is our personal approach to make safe RF applications surrounding the best site of SM unless there are close proximity to important structures such as the proximal conduction system and epicardial coronary arteries; locations that might require high precision to avoid collateral damage. Last but not the least, caution is warranted when relying on quantitative pace‐mapping automated algorithms. Despite technological improvements, ECG matching programs are imperfect and programable due to the discretion of the physician and/or the technician and as electrophysiologists we cannot forget to make visual comparison on the EP recording systems.
Study Limitations
Several limitations are related to our study. It is a small sample-sized, retrospective, single‐center study with a very short follow up, since one of our objectives were the feasibility of this approach. Second since there are several catheters operators’ difference between type of mapping catheter used, pace-mapping techniques and ablation approaches are common even when a prespecified protocol are made in the institution. Since the follow up was made with ECG monitoring for 5 to 10 minutes at 7 and 30 days after catheter ablation the 86% success rate may be lower in longer follow ups like at 6 as 12 months.
Conclusion
Catheter ablation of PVCs with exclusive Score Map software is feasible and is a very useful guide tool with good procedure and acute success rate with very low complications. When a minimum Score Map of more than 95% a high success rate was achieved. Markers of premature ventricular complexes recurrences were related to conscious sedation, female gender, left ventricle ablation and longer radiofrequency times.
References
- Morady F, Kadish AH, DiCarlo L, Kou WH, Winston S, et al. (1990) Long‐term results of catheter ablation of idiopathic right ventricular tachycardia. Circulation 82(6): 2093‐2099.
- Coggins DL, Lee RJ, Sweeney J, Chein WW, Van Hare G, et al. (1994) Radiofrequency catheter ablation as a cure for idiopathic tachycardia of both left and eight ventricular origin. J Am Coll Cardiol 23(6): 1333‐1341.
- Klein LS, Shih HT, Hackett FK, Zipes DP, Miles WM (1992) Radiofrequency catheter ablation of ventricular tachycardia in patients without structural heart disease. Circulation 85(5): 1666‐1674.
- Latchamsetty R, Yokokawa M, Morady F, Kim HM, Mathew S, et al. (2015) Multicenter outcomes for catheter ablation of idiopathic premature ventricular complexes. JACC Clin Electrophysiol 1(3): 116‐123.
- de Bakker JM, van Capelle FJ, Janse MJ, Wilde AA, Coronel R, et al. (1988) Reentry as a cause of ventricular tachycardia in patients with chronic ischemic heart disease: electrophysiologic and anatomic correlation. Circulation 77(3): 589‐606.
- de Bakker JMT, van Capflle FJL, Janse MJ, van Hemel NM, Hauer RN, et al. (1991) Macroreentry in the infarcted human heart: The mechanism of ventricular tachycardia with a “focal” activation pattern. J Am Coll Cardiol 18(4): 1005‐1014.
- Downar E, Harris L, Mickleborough LL, Shaikh N, Parson ID (1988) Endocardial mapping of ventricular tachycardia in the intact human ventricle: evidence for reentrant mechanisms. J Am Coll Cardiol 11(4): 783‐791.
- Shirai Y, Liang JJ, Santangeli P, Supple GE, Riley MP, et al. (2019) Catheter ablation of premature ventricular complexes with low intraprocedural burden guided exclusively by pace-mapping. J Cardiovasc Electrophysiol 1‐8.
- Capulzini L, Vergara P, Mugnai G, Salghetti F, Abugattas JP, et al. (2019) Acute and one year outcome of premature ventricular contraction ablation guided by contact force and automatedpacemapping software. J Arrhythm 35(3): 542-549.
- Steyers CMIII, Sodhi S, Faddis MN, Cooper DH, Cuculich PS, Noheria A (2019) Ablation using 3D maps adjusted for spatial displacement of premature ventricular complexes relative to sinus beats: Improving precision by correcting for the shift. J Cardiovasc Electrophysiol 1‐7.
- Noheria A, Buescher TL, Asirvatham SJ (2013) Electroanatomic mapping for catheter ablation of cardiac arrhythmias. In: Asirvatham SJ, Cha Y‐M, Friedman PA, eds. Mayo Clinic Electrophysiology Manual. 1st ed. Oxford, UK: Oxford University Press pp.75‐84.
- Movsowitz C, Schwartzman D, Callans DJ, Preminger M, Zado E, et al. (1996) Idiopathic right ventricular outflow tract tachycardia: narrowing the anatomic location for successful ablation. Am Heart J 131(5): 930‐936.
- Rodriguez LM, Smeets JLRM, Timmermans C, Wellens HJJ (1997) Predictors for successful ablation of right‐ and left‐sided idiopathic ventricular tachycardia. Am J Cardiol 79(3): 309‐314.
- Gerstenfeld EP, Dixit S, Callans DJ, Rajawat Y, Rho R, et al. (2003) Quantitative comparison of spontaneous and paced 12‐lead electrocardiogram during right ventricular outflow tract ventricular tachycardia. J Am Coll Cardiol 41(11): 2046‐2053.
- Baser K, Bas HD, Yokokawa M, Latchamsetty R, Morady F, et al. (2014) Infrequent intraprocedural premature ventricular complexes: implications for ablation outcome. J Cardiovasc Electrophysiol 25(10): 1088-1092.
- Aliot EM, Stevenson WG, Almendral-Garrote JM, Bogun F, Calkins CH, et al. (2009) Rhythm Association (EHRA); Registered Branch of the European Society of Cardiology (ESC); Heart Rhythm Society (HRS); American College of Cardiology (ACC); American Heart Association (AHA). EHRA/HRS Expert Consensus on Catheter Ablation of Ventricular Arrhythmias: developed in a partnership with the European Heart Rhythm Association (EHRA), a Registered Branch of the European Society of Cardiology (ESC), and the Heart Rhythm Society (HRS). Heart Rhythm 6(6): 886-933.
- (2016) EnSiteTM AutoMap Module. Instructions for Use. Jude Medical. One St. Jude Medical Drive St. Paul, MN. 2016.
- Ministry of Health - MS National Agency of Sanitary Surveillance - ANVISA RESOLUTION OF THE BOARD OF DIRECTORS - DRC No. 9, OF FEBRUARY 20, 2015.
- Rickham PP (1964) Human experimentation. Code of ethics of the World medical association. Declaration of Helsinki. Br Med J 2(5402): 177. Steinbach K, Glocar D, Weber H, Joscowicz G, Kaindl F (1982) Frequency and Variability of Ventricular Premature Contractions-The Influence of Heart Rate and Circadian Rhythms. Pacing Clin Electrophysiol 5(1): 38-51.
- Azegami K, Wilber DJ, Arruda M, Lin AC, Denman RA (2005) Spatial resolution of pacemapping and activation mapping in patients with idiopathic right ventricular outflow tract tachycardia. J Cardiovasc Electrophysiol 16(8): 823‐829.
- Bogun F, Taj M, Ting M, Kim HM, Reich S, et al. (2008) Spatial resolution of pace mapping of idiopathic ventricular tachycardia/ectopy originating in the right ventricular outflow tract. Heart Rhythm 5(3): 339‐344.
- Shirai Y, Goya M, Isobe M, Hirao K (2015) Preferential pathway pacing within the aortic sinus of valsalva: strong evidence for the existence of preferential conduction with different exit sites traversing the ventricular septum. J Cardiovasc Electrophysiol 26(7): 805‐808.
- Yamada T, Murakami Y, Yoshida N, Okada T, Shimizu T, et al. (2007) Preferential conduction across the ventricular outflow septum in ventricular arrhythmias originating from the aortic sinus cusp. J Am Coll Cardiol 50(9): 884‐891.
- Anter E, Frankel DS, Marchlinski FE, Dixit S (2012) Effect of electrocardiographic lead placement on localization of outflow tract tachycardias. Heart Rhythm 9(5): 697‐703.
- Li A, Davis JS, Wierwille J, Herold K, Morgan D, et al. (2017) Relationship between distance and change in surface ECG morphology during pacemapping as a guide to ablation of ventricular arrhythmias: implications for the spatial resolution of pacemapping. Circ Arrhythm Electrophysiol 10(1): e004447.






























