Intravascular Ultrasound (IVUS) for Deep Venous Work - Want or Need?
MR Toh1, K Damodharan2, MNHH Lim1, CJQ Yap1, S Dubenec3, PJ Tosenovsky4 and TY Tang1,5
1 Department of Vascular Surgery, Singapore General Hospital, Singapore
2Department of Interventional Radiology, Singapore General Hospital, Singapore
3 Department of Vascular Surgery, Royal Prince Alfred Hospital, Sydney, Australia
4 Department of Vascular Surgery, Royal Perth Hospital, Australia
5 Duke NUS Graduate Medical School, Singapore
Submission: March 18, 2020; Published: April 02, 2020
*Corresponding author: TY Tang, Senior Consultant, Department of Vascular Surgery, Singapore General Hospital Level 5; Academia, 20 College Road, Singapore
How to cite this article:MR Toh, K Damodharan, MNHH Lim, CJQ Yap, S Dubenec, PJ Tosenovsky, TY Tang. Intravascular Ultrasound (IVUS) for Deep Venous Work - Want or Need?. J Cardiol & Cardiovasc Ther. 2020; 16(2): 555931. DOI: 10.19080/JOCCT.2020.16.555931
Editorial
Intravascular ultrasound (IVUS) is an invasive modality that facilitates the endoluminal visualization of blood vessels. Increasingly, IVUS has gained favour in the management of chronic venous disease, which is a highly prevalent condition, occurring in approximately 30% of the general population. Contributing factors include previous deep vein thrombosis and iliocaval venous compression. In particular, prolonged compression of the iliocaval system has been associated with more severe disease and higher treatment failure rates [1]. Conventional vein ligation and stripping are inadequate, and deep venous stenting is required to prevent disease recurrence.
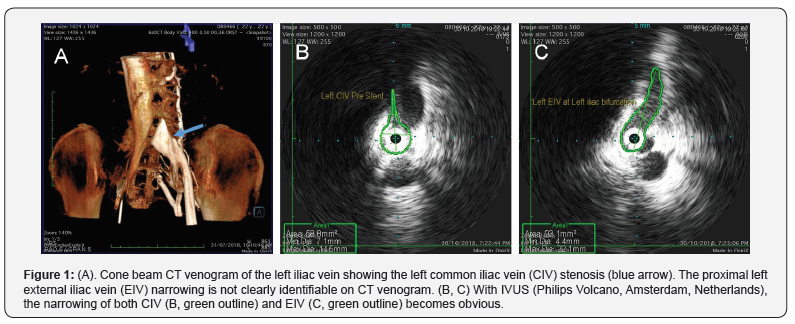
Initial diagnosis involves non-invasive and readily accessible tools such as ultrasound and computed tomography/magnetic resonance imaging. Unfortunately, these can only detect late signs such as venous insufficiency/reflux and obvious stenosis/occlusion. Early stenosis and endoluminal changes are only visualized on direct venography and IVUS. Direct venography with multiplanar views is the current gold standard imaging technique. However, a significant number of stenoses and morphological changes will still be missed (Figure 1) [2].
In a multicentric prospective study (VIDIO study) by Gagne et al., IVUS identified significant lesions missed on three-view venography in 26% of patients [2]. Treatment for 57 of 100 cases was revised after IVUS interrogation, with stents being placed in 54 patients who did not have appreciable lesions on venography alone [2]. The discrepancy in detection rates may be explained by the non-circular geometry of veins. Considering most venous narrowing are eccentric, vessel areas derived from diameters seen on venography would exceed the areas measured directly with IVUS [3]. Hence, the degree of stenosis on venography falls below that of IVUS by an average of 11% [2]. While multiple views can be obtained to improve the accuracy of venography, the operators and patients will be exposed to more radiation and contrast. IVUS is a more feasible alternative.
In addition, IVUS delineates the finer endoluminal features indicative of irreversible venous changes veins such as webs, trabeculation and scars [3]. Thrombi appear hyperechoic and thrombus age may be inferred from the degree of echogenicity [3]. Segments such as the ilio-caval bifurcation and ilio-femoral vein underlying the inguinal ligament, whilst not visible on frontal venographic projection, are easily visualized on IVUS [4]. IVUS allows the identification of hemodynamically significant lesions and dynamic venous compressions, which would otherwise be missed on static venography images [5]. Besides enhancing the diagnosis rates, IVUS avoids unnecessary stenting. Apparent stenoses from respiratory variation seen on venography can be excluded with IVUS, by asking the patient to take deep breaths or perform the Valsalva manoeuvre. In the VIDIO trial, three patients with significant lesions on venography were not stented based on their IVUS findings [2].
IVUS improves the precision of stent placement by determining the exact native vein diameter, diseased segment length and the appropriate landing sites. For adequate treatment, stents must be of sufficient length to extend into healthy venous segments both proximal and distal to the stenosis. In accordance with CIRSE standards, stents deployed under sole venography imaging should arbitrarily extend from the lesion by 5mm in both directions to circumvent the underestimation of stenosis length from venography [6]. The iliac confluence is a popular site for proximal landing zone. Unfortunately, the iliac confluence is often unclear on venography and the stent may be placed away from the desired proximal landing site by as much as an entire vertebral body [7]. This inaccuracy can compromise the intervention outcome. Insufficient placement into the inferior vena cava risks early postoperative stent occlusion while excess placement may compromise the blood flow in the contralateral iliac vein [6]. We recently discussed how IVUS can guide the accurate placement of stents across culprit lesions located near the ilio-caval bifurcation [8]. If needed, a contralateral puncture IVUS may be performed to aid accurate stent deployment [8].
IVUS also identifies tandem compression sites such as the external and internal iliac vein confluence, which may be compressed by the external iliac artery. These sites are obvious on IVUS but missed on venography. Clinically this would mean that a longer stent would be used to treat the lesion. Undersized stents can migrate and re-expose the stenosed sites, and in rare instances, embolize to the lungs [1]. IVUS prevents these complication rates by ensuring the correct size and placement of the stents. Once the stent is deployed, IVUS assesses the dilatation response and confirms adequate apposition of the stent to the venous wall [8]. Stagnant flow secondary to stent or outflow tract problems can be promptly picked up with IVUS and managed within the same setting [7].
Most of the IVUS applications in deep venous work involve non-occlusive chronic venous diseases. Nonetheless, IVUS is becoming increasingly relevant in thrombotic venous diseases. In acute deep vein thrombosis, IVUS helps to localize the guidewire by confirming the site of the thrombus and post-thrombectomy/ thrombolysis, IVUS reveals any venous wall irregularities and residual intraluminal thrombus that requires further treatment [9]. Unlike venography, IVUS can be used to image and treat acute deep vein thrombosis when contrast or radiation are contraindicated (e.g. during pregnancy) [9]. IVUS has been used with high success rates, for bedside insertion of inferior vena cava filters in critically ill patients who cannot be transported to an angiography suite [9].
IVUS has wide applications, but it is prudent to be aware of its limitations. Image artefacts such as acoustic shadowing from calcification, stent struts can obscure the underlying structures. Image quality is also impaired by rotational distortion artefacts created during manoeuvres in tortuous vessels, and multiple circular echoes from reverberating ultrasound beams [9].
With its superior sensitivity and resolution, IVUS has superseded venography in the diagnosis of chronic venous disease. Furthermore, it offers intraprocedural utility in guiding stent choice and placement. IVUS is integral to both the diagnosis and procedural management of chronic venous disease. Hence, we strongly recommend its routine use in deep vein work. Excitingly, ongoing research promises to augment the capability and applicability of IVUS. Currently under development, superharmonic IVUS imaging offers higher imaging depth, resolution and contrast-to-noise ratio, while accurate 3-D models of vessels and bifurcations better facilitate pre-procedural planning [10]. With these exciting developments on the horizon, IVUS may eventually become indispensable in deep venous work.
Author Contributions
MR Toh is the main author responsible for the drafting and editing of the manuscript. TY Tang envisaged the idea and helped draft and edit the final manuscript version. K Damodharan, CJQ Yap, S Dubenec, PJ Tosenovsky were involved in editing and contributing pictures to the manuscript.
References
- Raju S, Neglen P (2006) High prevalence of nonthrombotic iliac vein lesions in chronic venous disease: a permissive role in pathogenicity. J Vasc Surg 44(1): 136-144.
- Gagne PJ, Tahara RW, Fastabend CP, Dzieciuchowicz L, Marston W, et al. (2017) Venography versus intravascular ultrasound for diagnosing and treating iliofemoral vein obstruction. J Vasc Surg Venous Lymphat Disord 5(5): 678-687.
- Neglén P, Raju S (2002) Intravascular ultrasound scan evaluation of the obstructed vein. J Vasc Surg 35(4): 694-700.
- Neglén P, Hollis KC, Olivier J, Raju S (2007) Stenting of the venous outflow in chronic venous disease: Long-term stent-related outcome, clinical, and hemodynamic result. J Vasc Surg 46(5): 979-990.
- Chamarthy MR, Sutphin P, Anderson M, Reddick M, Kalva SP (2017) Evolving Concepts in the Diagnosis and Management of May–Thurner Syndrome. J Clin Interv Radiol ISVIR 01(01): 023-030.
- Mahnken AH, Thomson K, de Haan M, O'Sullivan GJ (2014) CIRSE standards of practice guidelines on iliocaval stenting. Cardiovasc Intervent Radiol 37(4): 889-897.
- Murphy E, Johns B, Alias M, Crim W, Raju S, et al. (2016) VESS25. Inadequacies of Venographic Assessment of Anatomic Variables in Iliocaval Disease. Journal of Vascular Surgery 63(6): 33S-34S.
- Tang T, Goh R, Damodharan K, Choke EC2, Chong TT, et al. (2019) RE:“Long-term follow-up of stenting across the ilio-caval confluence in patients with iliac venous lesions”: the value of using IVUS and a dedicated oblique venous stent for deep vein work involving the ilio-caval bifurcation. Journal of thrombosis and thrombolysis 47(2): 328-330.
- McLafferty RB (2012) The role of intravascular ultrasound in venous thromboembolism. Semin Intervent Radiol 29(1): 10-15.
- Marteslo JP, Makary MS, Khabiri H, et al. (2019) Intravascular Ultrasound for the Peripheral Vasculature-Current Applications and New Horizons. Ultrasound in med biol 46(2): 216-224.






























