Hypertension and Emergency Department Visits by Patients with Head and Neck Cancer
Marcelo Sandoval1, Srinivas R Banala1, Maria Teresa Cruz Carreras1, Demis N Lipe1, Sai Ching J Yeung1, Ehab Y Hanna2, Knox H Todd1,2, Kumar Alagappan1 and Cielito C Reyes Gibby1,3*
1Department of Emergency Medicine, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Boulevard, USA
2Department of Head and Neck Surgery, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Boulevard, USA
3Department of Biostatistics, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Boulevard, USA
Submission: March 19, 2020; Published: March 30, 2020
*Corresponding author: Cielito C Reyes-Gibby, Associate Professor, Department of Emergency Medicine, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Boulevard, Unit 1468, Houston, Texas 77030, USA
How to cite this article:Marcelo S, Srinivas R B, Maria T C C, Demis N L, Sai Ching J Y, et al. Hypertension and Emergency Department Visits by Patients with Head and Neck Cancer. J Cardiol & Cardiovasc Ther. 2020; 16(1): 555929. DOI: 10.19080/JOCCT.2020.15.555929
Abstract
Background: The Emergency Department (ED) is the safety net for unanticipated or undertreated health needs. Patients with cancer have been reported to be substantial users of ED resources, to be of higher acuity than others, and to have a longer length of stay. Patients with head and neck cancer live longer than patients with other types of cancer. Therefore, we assessed the extent to which epidemiological, behavioral, and clinical factors collected prior to treatment were associated with eventual ED visits in patients with head and neck cancer.
Methods: Questionnaires were administered at baseline, prior to cancer treatment. ED data were abstracted for up to 5 years follow up period from initial diagnosis and treatment of patients newly diagnosed with squamous cell carcinoma of the head and neck (HNSCC).
Results: Our sample comprised 969 patients. The earliest ED visit occurred within 1 week of diagnosis. As many as 513 patients had ≥1 ED visit and the mean time to first ED visit was 27 weeks (SD=2.3 weeks). The most frequent chief complaints were: pain (n=181; 35.3% [abdominal pain=16.3%, chest pain=7.5%]); fever (n=107; 20.9%); nausea/vomiting (n=64; 12.5%); weakness/fatigue (n=45; 8.8%). Multivariate logistic regression indicated that hypertension (OR=1.43, 95% CI=1.02–2.03; P=0.039), T-stage (OR=2.05, 95% CI=1.45–2.92; P<0.0001), and N-stage (OR=1.47, 95% CI=1.17–1.86; P<0.001) were significantly associated with ED visits.
Conclusion: To our knowledge, our study is the first to find a specific association between hypertension and ED visits in patients with HNSCC. Further research is needed to investigate possible reasons for the association between comorbidities such as hypertension and the need for emergent care, as well as to determine whether aggressive management of comborbid conditions during and after cancer therapy might reduce the likelihood of ED visits.
Keywords: Squamous cell carcinoma of the head and neck; Emergency; Pain; Depressed mood; Fatigue; Hypertension
Introduction
Emergency departments (EDs) are becoming important primary sites for the care of cancer-related complications. Patients with cancer have been reported to be substantial users of ED resources, to be of higher acuity than others, and to have a longer length of stay [1]. ED visits can result in hospitalizations and increased costs of care, cause breaks in ongoing cancer treatment, and negatively affect quality of life and overall survival [2-5]. ED visits are an important indicator of the quality of healthcare received by cancer patients.
Standard cancer therapies, including chemotherapy, radiotherapy, and surgery, can produce numerous short-term and long-term treatment-related adverse effects that may require emergency care [3]. Further, the malignant process and its progression can exacerbate common preexisting conditions [2]. Prompt diagnosis and appropriate treatment of these emergencies is essential to help restore a patient’s condition [2] and, possibly, to circumvent a life-threatening situation. The aging of the population, existing comorbidities, and the development of new therapeutic drugs and treatment strategies for malignant disorders increase the complexity of managing patients with cancer when they present to the ED. Understanding risk factors for eventual ED visits on the basis of baseline indicators-for example, preexisting comorbidities-could promote better management for these patients during cancer treatment and could help them avoid subsequent utilization of emergency care services.
In this study, we assessed the extent to which clinical, behavioral, and epidemiological factors reported before commencement of cancer treatment were independent risk factors for eventual ED visits. Our sample was a group of treatment-naïve patients with newly diagnosed squamous cell carcinoma of the head and neck (HNSCC), which includes cancers of the pharynx, larynx, and oral cavity [6] who presented for treatment at a tertiary cancer center. We selected this population because patients with HNSCC receive intense outpatient radiation and chemotherapy and may undergo surgical interventions (resection, tracheostomy, feeding gastrostomy) [7]; they also tend to live longer than patients with other types of cancer [8]. Treatment side effects, debilitating functional impairment, and complex psychosocial issues may develop for weeks to years after diagnosis and may necessitate visits to the ED.
Although health outcomes in patients with HNSCC are known to vary by extent of disease, little is known about prognostic factors for ED visits. To our knowledge, this is the first study to include a comprehensive assessment of potential risk factors (clinical, epidemiological, and behavioral factors) for ED visits in this population. This is an important aspect of efforts to integrate the ED into the spectrum of care of cancer survivors [1].
Materials and Methods
Study Setting and Population: The study population included all patients with newly diagnosed HNSCC who were initially treated in the Head & Neck Center of a tertiary cancer center between 2006 and 2009. Follow-up data were available for up to 5 years.
Ethical Approval: This study was conducted according to a clinical research protocol approved by our Institutional Review Board. All procedures adhered to its guidelines and regulations, in accordance with the Declaration of Helsinki Ethical Principles for Medical Research Involving Human Subjects and with US Health Insurance Portability and Accountability Act regulations.
Outcome Variables
The primary binary outcome variable was at least one ED visit (yes/no). The descriptive analyses included additional outcome variables such as chief complaints at the time of ED presentation, time to first ED visit, and frequency of ED visits during the followup period. Patients were followed for up to 5 years or until death.
Independent Variables
All independent variables were collected at the time of registration in the Head & Neck Center (baseline), prior to cancer treatment. The questionnaire was developed by an interdisciplinary team of scientists representing the areas of epidemiology, medical oncology, behavioral science among others. The overarching goal was to understand the epidemiology of the different types of cancers and the underlying factors associated with, and risk factors for, cancer, cancer progression, and survival outcomes. Many questionnaire items were considered, but the committee was very cognizant of patient burden, and the final set of questions was decided through consensus [9]. Clinical data including stage of disease were abstracted from patients’ charts.
Epidemiological factors: Epidemiological variables included age at the time of cancer diagnosis, sex, and self-reported race/ ethnicity (non-Hispanic white, non-Hispanic black, or Hispanic). We excluded patients from other race categories due to small sample size. Patient-reported comorbidities included heart disease, stroke, hypertension, diabetes, and lung disease.
Clinical factors: Cancer-related variables included TNM (T=primary tumor size, extent, or depth of penetration; N=lymph node involvement; M=presence of metastasis) staging.
Behavioral factors: Behavioral variables included smoking and alcohol consumption. Smoking was categorized as never smoker, former smoker, or current smoker. Alcohol intake was classified as never, social, moderate, or heavy. We defined heavy alcohol use as ≥4 drinks per day, irrespective of sex, and moderate alcohol use as >14 drinks per week for males and >7 drinks per week for females, but in either case <4 drinks per day [10].
Patient-Reported Outcomes: Symptoms assessed at baseline (pretreatment) included pain, depressed mood, and fatigue--the most common side effects of cancer and its treatment [9,11-13]. Baseline pain was assessed with two questions: “Have you experienced pain in the last week?” (yes/no) and “Circle the number that best describes the pain you are having” (rated on an 11-point numeric scale, with 0=no pain and 10=pain as bad as you can imagine). The 0–10 scale is a recommended standard for pain assessment in clinical studies of pain [14]. We used the National Comprehensive Cancer Network’s cutoff score of ≥7 on the 0–10 scale to indicate severe pain [15].
Two items from the SF-12, a validated, widely used measure of quality of life in patients with cancer [16-19], were used to assess depressed mood (“During the past 4 weeks, have you felt downhearted and blue?”) and fatigue (“During the past 4 weeks, did you have a lot of energy?”). These items were rated on a 6-point Likert scale; patients responding “most of the time” or “all of the time” were considered to have severe depressed mood or fatigue, respectively [20].
Dependent Variables
Emergency Department Data: Our institution’s ED has 43 beds and is staffed 24 hours a day, 7 days a week. Information on all patients who visit the ED is collected in a database maintained by the Department of Emergency Medicine. Initiated in 2006, the database includes demographic information, type of cancer, and primary and secondary presenting symptoms (chief complaints). We reviewed ED data for up to 5 years from diagnosis and treatment for each patient
Data Analysis
Descriptive statistics were used to summarize patient characteristics. Patients were coded as “0” if they had no ED visits and “1” if they had at least one ED visit. Follow-up time was defined as time from diagnosis to first ED visit (for those who presented to the ED at least once), or to the date of abstraction (for individuals with no ED visits) or death (for individuals who died during the follow-up period but did not present to the ED).
Univariate and multivariate logistic regression analyses were used to estimate the strength of association with ED visits for the variables. Factors found to be significant (P<0.20) in the univariate analysis were included in the multivariate model; a P value of 0.20 was used because the traditional value (P<0.05) often does not identify variables shown to be important in the literature [21]. Further variable selection in the multivariate model was conducted using backward elimination. To obtain the most parsimonious model, only variables with P values <0.05 were included in the final model. All statistical analyses were performed using SPSS software (SPSS Inc., Chicago, IL). All statistical tests were 2-sided.
Results
Study Population
A total of 969 patients with HNSCC comprised our sample; of these, 274 had cancer of the pharynx, 176 had cancer of the larynx, and 519 had cancer of the oral cavity. The mean age for the total sample was 59±11 years, and most of the patients were men (78.6%; 762/969).
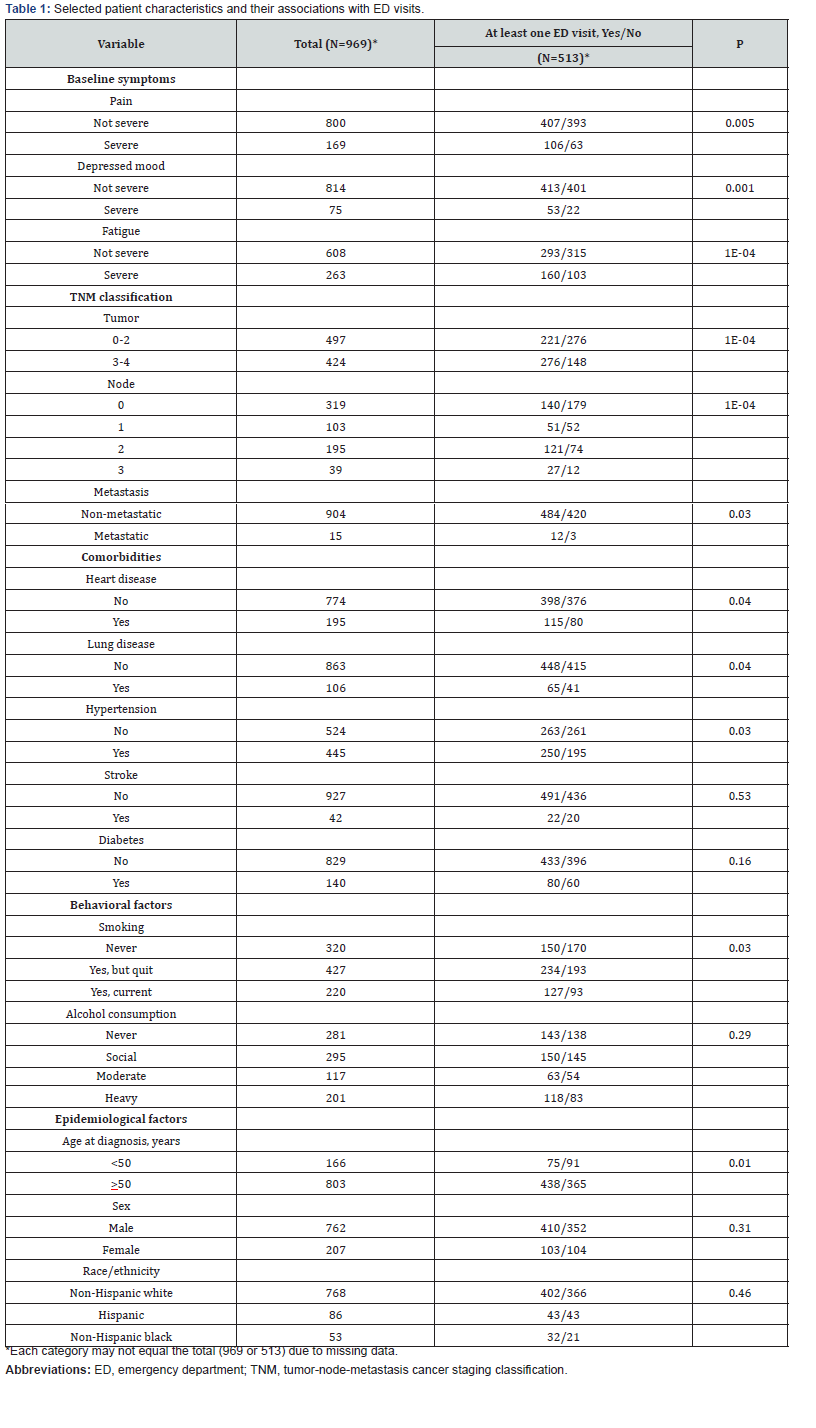
Selected patient characteristics are shown in Table 1. The most commonly reported comorbid conditions at baseline were hypertension (45.9%; 445/969), followed by heart disease (20.1%; 195/969) and diabetes (14.4%; 140/969). Approximately one in four participants were current smokers (22.8%; 220/967) or heavy drinkers (22.5%; 201/894). At baseline, severe pain (rated ≥7 on the 0-10 scale) was reported by 17.4% of patients (169/969); 8.4% (75/889) reported depressed mood and 30.2% (263/871) reported severe levels of fatigue.
ED Visits
We found that 513 (53%) of the patients visited the ED at least once after cancer treatment during the follow-up period. The most frequent chief complaints at the first ED visit were: pain (n=181; 35.3% [abdominal pain=16.3%, chest pain=7.5%]); fever (n=107; 20.9%); nausea/vomiting (n=64; 12.5%); weakness/ fatigue (n=45; 8.8%); bleeding (n=35; 6.8%); shortness of breath (n=30; 5.8%); and change in mental status (n=13; 2.5%).
The mean and median number of ED visits for the entire sample were 1.5 visits (SD=2.3 visits) and 1 visit (range, 0–16 visits), respectively. The first ED visit occurred during the first week after diagnosis and presentation to the cancer center. The mean time to first ED visit for the entire sample was 27 weeks (SD=2.3 weeks). Table 1 shows that ED visit varied significantly by comorbidities (heart disease, lung disease, hypertension), smoking status, age, baseline symptom severity (pain, fatigue, depressed mood) and disease stage (TNM).
Factors Associated with ED Visits
Table 2 shows the results of the univariate and multivariate analyses. Univariate analysis revealed the following significant factors for ED visits: extent of disease, as evidenced by T, N, and M staging as separate variables; certain clinical comorbidities, including heart disease, lung disease, and hypertension; and smoking. Patients with severe levels of pain, depressed mood, or fatigue at baseline were also more likely to visit the ED than were patients who did not report having severe levels of these symptoms.
In multivariate analyses, we assessed the extent to which factors from the univariate model influenced ED visits, adjusting for time to first ED visit. The multivariate models indicated that hypertension (OR=1.43, 95% CI=1.02–2.03; P=0.039) , T-stage (OR=2.05, 95% CI=1.45-2.92; P<0.0001), and N-stage (OR=1.47, 95% CI=1.17–1.86; P<0.001) were significant factors for ED visits.
Discussion
There are more than 14 million cancer survivors in the United States and, of these, as many as 5 million are still within 5 years of their primary diagnosis [22]. Although advances in cancer treatment have led to increases in survival, the early and late toxicities of cancer treatment can be debilitating enough to require medical care [3,23,24] and a visit to the ED. Preexisting comorbid conditions, such as hypertension, can not only predispose a patient to ED utilization earlier during cancer treatment, but can also put the patient at risk for worsening of disease. Because cancer treatment is often provided on an outpatient basis, understanding factors that are associated with ED presentation within the cancer population has clinical significance.
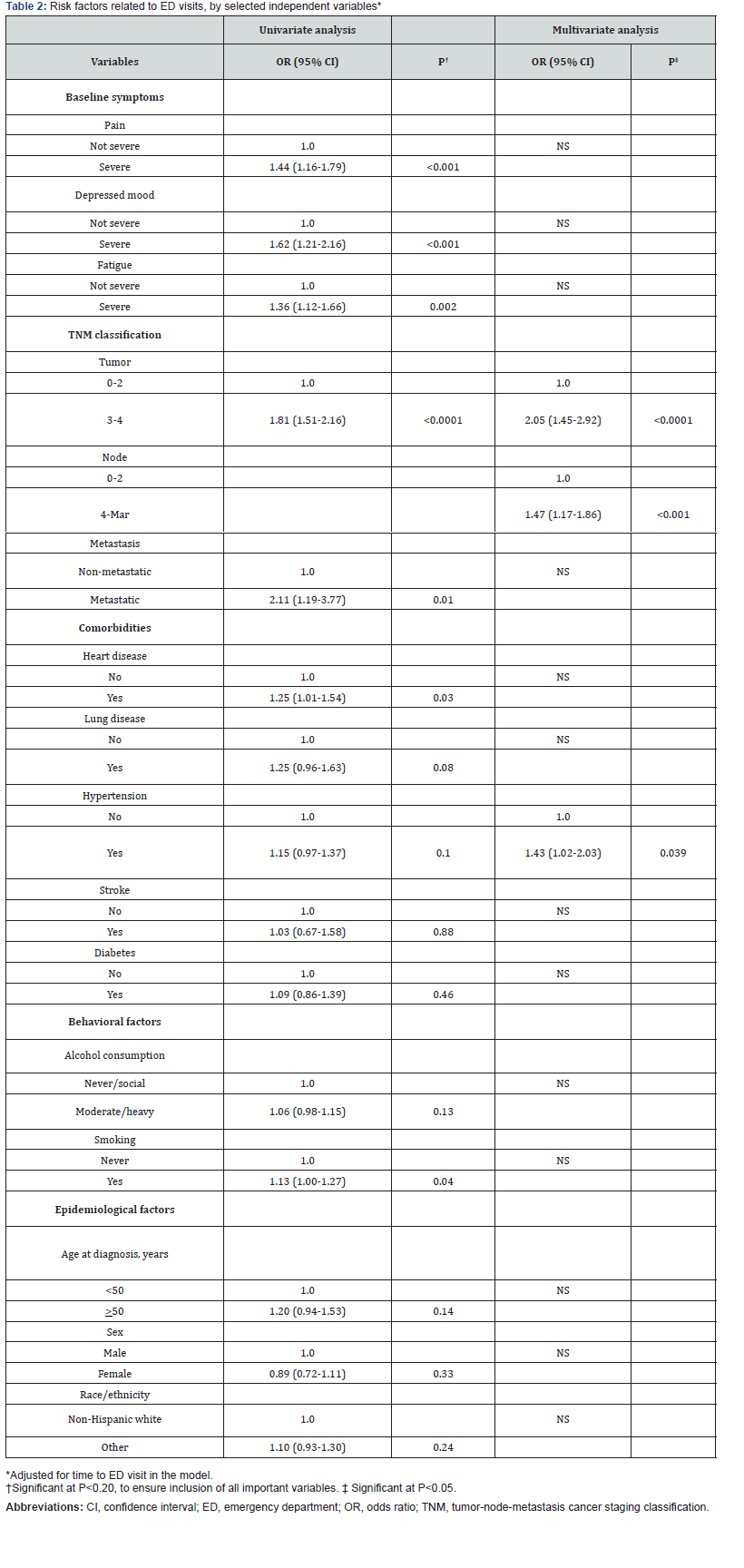
In our sample, hypertension as a pretreatment comorbidity was one of only two significant risk factors for ED visits in both univariate and multivariate analysis (the other being TN status). However, little is known about the association between hypertension, cancer, and ED visits, even though hypertension is the most common comorbidity seen in patients with malignant conditions [25]. Hypertension is a well-established risk factor for chemotherapy-induced cardiotoxicity [26]; moreover, certain chemotherapeutic agents, such as the vascular signaling pathway inhibitors, are known to cause hypertension and can potentially worsen preexisting disease. These drugs inhibit angiogenesis and play a key role in cancer-targeted therapy. Surgery or radiation therapy that involves the head or neck can lead to baroreflex failure and to associated difficult-to-treat labile hypertension and hypertensive crisis [27]. Poorly controlled hypertension can cause severe symptoms that influence cancer management, thereby potentially increasing the number of ED visits [26].
Assessment of comorbid hypertension at baseline, prior to initiation of cancer treatment, has been recommended by the panel of Investigational Drug Steering Committee of the US National Cancer Institute [26], as a way to minimize the risk of end-organ damage, enable continuation of cancer therapy, and prevent other complications. Therefore, patients with preexisting hypertension presenting to the ED with newly diagnosed cancer should be assessed for end-organ damage, worsening hypertension, and cardiotoxicity. In our study of treatment-naïve patients, 7.5% of patients presented to the ED with chest pain and an additional 8.3% presented with shortness of breath or altered mentation. Those patients presenting with hypertension and not currently on cancer treatment should be carefully assessed, and treatment of their hypertension should be considered.
Patients with HNSCC may also be especially prone to dehydration because of reduced oral intake resulting from dysphagia, the severity of which depends on the size and location of the lesion. Thus, awareness on the use of antihypertensive medications, especially diuretics is important since these medications can worsen dehydration and can induce hypotension in a dehydrated patient.
Baseline disease stage (T stage and N stage) was the other important factor influencing the probability of an ED visit. This is expected, as patients with advanced disease often present with symptoms indicative of disease progression; further, the symptoms experienced by patients with advanced disease are of increased severity, which can make emergency care necessary [28].
Pain and fever were two of the most frequent primary chief complaints reported by our sample of HNSCC patients at the time of ED presentation, a finding that is consistent with results from other studies. For example, in 2015, Tang et al. [29] used nationwide population-based data to investigate the chief reasons for ED visits made by HNSCC patients in Taiwan. The study revealed that pain was one of three principal complaints, alongside respiratory distress and gastrointestinal issues, for ED visits in that patient population. Tsai et al. [30] also found that pain was the most common reason that patients with cancer visit the ED. This finding was corroborated in a study conducted at a tertiary care center in Brazil by Kraft Rovere et al. [28], who reported that the pain was the most common complaint of patients with head and neck cancer, who comprised 9% of all emergency visits by cancer patients. We found that most of the patients with HNCSS in our sample who presented to the ED with pain had abdominal or chest pain, similar to the findings by Tsai et al. [30].
Fever is not unusual in patients with cancer, who have weakened immune systems that make them prone to infection. In our study, we did not distinguish those who came to the ED with neutropenic fever from those who did not. Nonetheless, neutropenia is a concern for many of the HNSCC patients who come to the ED with fever. A study by Vidal et al. [31] noted that infection is the principal cause of about two-thirds of cases of fever with prolonged neutropenia seen in patients with cancer. Infectious Diseases Society of America 2010 guidelines [32] for treating neutropenic fever state that all patients should be treated with broad-spectrum antibiotics within 2 hours of presentation. Stable, well-appearing patients with a solid tumor may be discharged with oral antibiotics and close follow-up on an outpatient basis; conversely, unstable or ill-appearing patients with solid tumors and all patients with liquid tumors (leukemia, lymphoma) require immediate hospital admission when neutropenic fever is present. require hospitalization. Patients who have had stem cell transplants are considered immunosuppressed and should be treated much like neutropenic patients.
Our study had limitations. We did not include type of cancer treatment as a covariate. However, treatment is driven by extent of disease and is therefore associated with tumor stage, which was assessed in this study (hence, high multicollinearity). The study was also limited to patients with HNSCC at one tertiary cancer center; it is possible that additional visits were made to EDs other than ours. Finally, only very few patients of Asian/Pacific Islander or American Indian/Alaska Native racial origin were available for recruitment, limiting the generalizability of our findings. Thus, additional studies are needed to validate our findings.
Conclusion
The ED is the safety net for unanticipated or undertreated health needs. To our knowledge, our study is the first to find a specific association between hypertension and ED visits in patients with HNSCC. Further research is needed to investigate possible reasons for the association between comorbidities such as hypertension and the need for emergent care, as well as to determine whether aggressive management of comborbid conditions during and after cancer therapy might reduce the likelihood of ED visits.
Acknowledgments
The authors acknowledge Jeanie F. Woodruff, BS, ELS, for editorial assistance and Valda D. Page, MPH, BS, for data management.
Funding: This work was supported by the National Institute of Dental and Craniofacial Research of the National Institutes of Health (NIH) [grant number R01 DE022891; PI: Cielito C. Reyes- Gibby]; the National Cancer Institute of the NIH [grant number P30 CA016672, MD Anderson Cancer Center Support Grant; PI: Peter Pisters), and the MD Anderson Program in Oncologic Emergency Medicine [PI: Cielito C. Reyes-Gibby]. The NIH had no role in the conduct or reporting of the study; the content of this report is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Author’ Contributions: SRB, S-CJY, KHT, and CCR: study concept and design. SRB: acquisition of data/chart review; SRB, S-CJY, and CCR: analysis and interpretation of data; MS, SRB, MTCC, S-CJY, and CCR: drafting of the manuscript; MS, MTCC, DNL, S-CJY, EYH, KHT, KA, and CCR: critical revision of the manuscript for important intellectual content; S-CJY and CCR: statistical expertise; KHT and CCR: obtained funding; EYH, KHT, KA: administrative, technical, or material support; KHT and KA: study supervision. CCR takes responsibility for the paper as a whole.
Competing Interests: MS reports no conflicts of interest. SRB reports no conflicts of interest. MTCC reports no conflicts of interest. S-CJY reports research funding from DepoMed, Newark, CA and Bristol-Myers Squibb (ARISTA-USA program). EYH reports no conflicts of interest. KHT reports no conflicts of interest. KA reports no conflicts of interest. CCR reports no conflicts of interest.
References
- Hsu J, Donnelly JP, Moore JX, Meneses K, Williams G, et al. (2018) National characteristics of Emergency Department visits by patients with cancer in the United States. Am J Emerg Med 36(11): 2038-2043.
- Sadik M, Ozlem K, Huseyin M, AliAyberk B, Ahmet S, et al. (2014) Attributes of cancer patients admitted to the emergency department in one year. World J Emerg Med 5(2): 85-90.
- Vandyk AD, Harrison MB, Macartney G, Ross-White A, Stacey D (2012) Emergency department visits for symptoms experienced by oncology patients: a systematic review. Support Care Cancer 20(8): 1589-1599.
- Bozdemir N, Eray O, Eken C, Şenol Y, Artaç M, et al. (2009) Demographics, clinical presentations and outcomes of cancer patients admitted to the emergency department. Turk J Med Sci 39(2): 235-240.
- Porta M, Fernandez E, Belloc J, Malats N, Gallén M, et al. (1998) Emergency admission for cancer: a matter of survival? Br J Cancer 77(3): 477-484.
- Dansky Ullmann C, Harlan LC, Shavers VL, Stevens JL (2012) A population-based study of therapy and survival for patients with head and neck cancer treated in the community. Cancer 118(18): 4452-4461.
- Pfister DG, Spencer S, Brizel DM, Burtness B, Busse PM, et al. (2015) Head and Neck Cancers, Version 1. J Natl Compr Canc Netw 13(7): 847-855.
- (2016) American Cancer Society. Cancer Facts & Figures 2016 Atlanta: American Cancer Society.
- Reyes-Gibby CC, Anderson KO, Merriman KW, Todd KH, Shete SS, et al. (2014) Survival patterns in squamous cell carcinoma of the head and neck: pain as an independent prognostic factor for survival. J Pain 15(10): 1015-1022.
- (2017) National Institute on Alcohol Abuse and Alcoholism. Alcohol & Your Health: Drinking Levels Defined Bethesda MD.
- Reyes-Gibby CC, Swartz MD, Yu X, Wu X, Yennurajalingam S, et al. (2013) Symptom clusters of pain, depressed mood, and fatigue in lung cancer: assessing the role of cytokine genes. Support Care Cancer 21(11): 3117-3125.
- Patrick DL, Ferketich SL, Frame PS, et al. (2004) National Institutes of Health State-of-the-Science Conference Statement: symptom management in cancer: pain, depression, and fatigue, July 15-17, 2002. J Natl Cancer Inst Monogr 32: 9-16.
- Scharpf J, Karnell LH, Christensen AJ, Funk GF (2009) The role of pain in head and neck cancer recurrence and survivorship. Arch Otolaryngol Head Neck Surg 135(8): 789-794.
- Caraceni A, Cherny N, Fainsinger R, Kaasa S, Poulain P, et al. (2002) Pain measurement tools and methods in clinical research in palliative care: recommendations of an Expert Working Group of the European Association of Palliative Care. J Pain Symptom Manage 23(3): 239-255.
- Swarm RA, Abernethy AP, Anghelescu DL, Benedetti C, Buga S, et al. (2013) Adult cancer pain. J Natl Compr Canc Netw 11(8): 992-1022.
- Ware J Jr, Kosinski M, Keller SD (1996) A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care 34(3): 220-233.
- Vilaseca I, Chen AY, Backscheider AG (2006) Long-term quality of life after total laryngectomy. Head Neck 28(4): 313-320.
- Wei JT, Dunn RL, Litwin MS, Sandler HM, Sanda MG (2000) Development and validation of the expanded prostate cancer index composite (EPIC) for comprehensive assessment of health-related quality of life in men with prostate cancer. Urology 56(6): 899-905.
- Wettergren L, Björkholm M, Axdorph U, Langius-Eklöf A (2004) Determinants of health-related quality of life in long-term survivors of Hodgkin's lymphoma. Qual Life Res 13(8): 1369-1379.
- Reyes-Gibby CC, Anderson KO, Shete S, Bruera E, Yennurajalingam S (2012) Early referral to supportive care specialists for symptom burden in lung cancer patients: a comparison of non-Hispanic whites, Hispanics, and non-Hispanic blacks. Cancer 118(3): 856-863.
- Bendel RB, Afifi AA (1977) Comparison of stopping rules in forward stepwise regression. J Am Stat Assoc 72(357): 46-53.
- DeSantis CE, Lin CC, Mariotto AB, Siegel RL, Stein KD, et al. (2014) Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin 64(4): 252-271.
- Chikarmane SA, Khurana B, Krajewski KM, Shinagare AB, Howard S, et al. (2012) What the emergency radiologist needs to know about treatment-related complications from conventional chemotherapy and newer molecular targeted agents. Emerg Radiol 19(6): 535-546.
- Stone JB, DeAngelis LM (2016) Cancer-treatment-induced neurotoxicity--focus on newer treatments. Nat Rev Clin Oncol 13(2): 92-105.
- Piccirillo JF, Tierney RM, Costas I, Grove L, Spitznagel EL Jr (2004) Prognostic importance of comorbidity in a hospital-based cancer registry. JAMA 291(20): 2441-2447.
- Mouhayar E, Salahudeen A (2011) Hypertension in cancer patients. Tex Heart Inst J 38(3): 263-265.
- Ketch T, Biaggioni I, Robertson R, Robertson D (2002) Four faces of baroreflex failure: hypertensive crisis, volatile hypertension, orthostatic tachycardia, and malignant vagotonia. Circulation 105(21): 2518-2523.
- Kraft Rovere R, Dagnoni C, E GC, E Dde O, Figueira FC, et al. (2012) Profile of cancer patients treated at the emergency room of a tertiary cancer care centre in southern Brazil. Klin Onkol 25(6): 452-456.
- Tang PL, Cheng JS, Huang WC, Chang HS, Chen HC (2015) Why do head and neck cancer patients visit the emergency department? Am J Emerg Med 33(8): 1102-1105.
- Tsai SC, Liu LN, Tang ST, Chen JC, Chen ML (2010) Cancer pain as the presenting problem in emergency departments: incidence and related factors. Support Care Cancer 18(1): 57-65.
- Vidal M, Ferrer A, Serrano S, Tobeña M, Pajares I, et al. (2009) Fever in cancer patients as a cause of attendance in emergency room. American Society of Clinical Oncology 45th Annual Meeting, Orlando FL, May 29-Jun 2, J Clin Oncol, 2009.
- Freifeld AG, Bow EJ, Sepkowitz KA, Boeckh MJ, Ito JI, et al. (2011) Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 Update by the Infectious Diseases Society of America. Clin Infect Dis 52(4): 427-431.






























