Hypertensive Cardiomyopathy: Diagnostic Approach and Clinical Differentiation from Hypertrophic Cardiomyopathy
Galaleldin Nagib Elkilany1*, Sherif Baath Allah2, Jan Fedacko3, Jaipaul Singh4, Ram B Singh5, Navin C Nanda6 and Mai Salama7
1 1,7Professor and Consultant of Cardiology (SCU) Tanta University, Egypt & Assistant Clinical Professor at Gulf Medical University, Ajman, UAE
2RAK Medical and Health Science University
3Kosice Medical University, Slovakia
4University of Central Lancashire, Preston, UK,
5Halberg Hospital and Research Institute, Moradabad, India
6Alabama University at Birmingham, AL, USA
Submission: December 03, 2019; Published: December 20, 2019
*Corresponding author: Galaleldin Nagib Elkilany, Professor and Consultant of cardiology, SCU - Tanta University, Egypt, 132 Holly Drive, LaPlace, LA, 70068, USA
How to cite this article:Galaleldin N E, Sherif B A, Jan F, Jaipaul S, Ram B S, et al. Hypertensive Cardiomyopathy: Diagnostic Approach and Clinical Differentiation from Hypertrophic Cardiomyopathy. J Cardiol & Cardiovasc Ther. 2019; 15(4): 555919. DOI: 10.19080/JOCCT.2019.15.555919
Abstract
Hypertensive Cardiomyopathy (HTN-CM) is a structural cardiac disorder generally accompanied by concentric or eccentric Left Ventricular Hypertrophy (LVH) associated with diastolic or/and systolic dysfunction in patients with persistent systemic hypertension. It occurs in the absence of other cardiac diseases capable of causing myocardial hypertrophy or cardiac dysfunction. Long standing arterial hypertension (HTN) leads to structural and functional myocardial abnormalities resulting in myocardial ischemia, fibrosis, and hypertrophy. HTN-CM is predominantly a disease of impaired relaxation rather than impaired contractility, although subtle myocardial systolic abnormalities could be detected recently by Global Longitudinal Systolic Strain (GLS) Speckle Tracking Echocardiography (STE). Importantly, the accompanying LVH is itself a risk factor for mortality and morbidity and is considered an independent predictor for Sudden Cardiac Death (SCD). Therefore, early detection of LVH development in patients with Hypertensive Hypertrophic Cardiomyopathy (HTN-CM) is crucial for optimal treatment. In addition to pathological findings, echocardiography and cardiac magnetic resonance imaging are ideal tools for the diagnosis of HTN-CM and can differentiate it from Hypertrophic Cardiomyopathy (HCM). Timely diagnosis of this condition and utilization of appropriate treatment are required to improve morbidity and mortality in hypertensive patients. This review presents an overview of utilization of multidisciplinary imaging modalities approach for proper diagnosis of HTN-CM and its differentiation from HCM. Relevant article highlighted key points in differentiation of HTN-CM from HCM and the effects of hypertension on cardiac hypertrophy and heart failure development are discussed in clinical case study.
Keywords: Hypertension; Cardiomyopathy; Heart Failure; Sudden Cardiac Death; Echocardiography; Strain and Speckle tracking Echocardiography; Cardiac Magnetic Resonance Imaging
Abbreviations: HCM: Hypertrophic Cardiomyopathy; HHD: Hypertensive Heart Disease; LVH: Left Ventricular Hypertrophy; CMR: Cardiac Magnetic Resonance; HTN-CM: Hypertensive Cardiomyopathy; Left Ventricular Outflow Tract; ACE: Angiotensin-Converting-Enzyme; RAAS: Renin Angiotensin Aldosterone System; ECG: Electrocardiography; Diastolic Dysfunction; TDI: Tissue Doppler Imaging; GLS: Global Longitudinal Systolic; LGE: Late Gadolinium Myocardial Enhancement; HOCM: Hypertrophic Obstructive Cardiomyopathy
Introduction
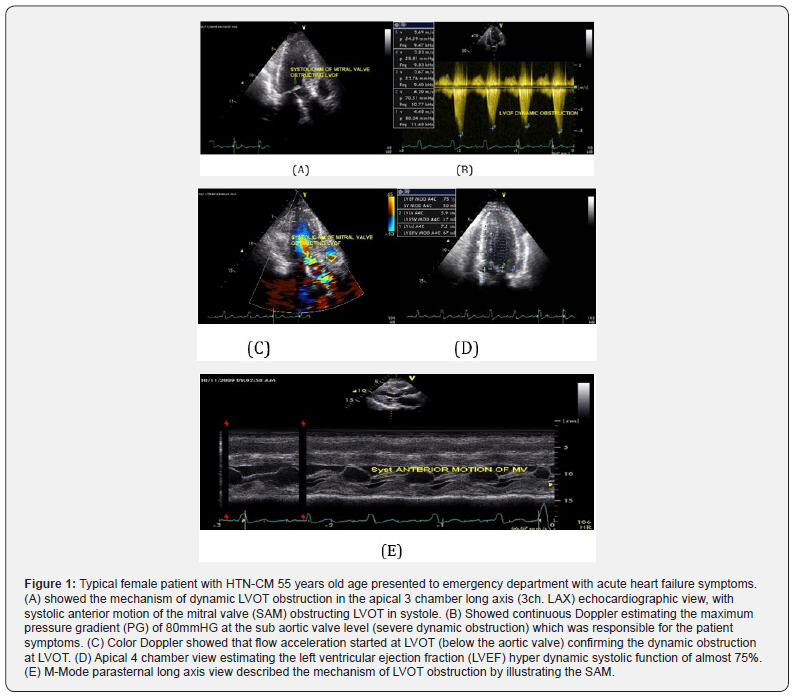
Both hypertrophic cardiomyopathy (HCM) and hypertensive heart disease (HHD) present left ventricular hypertrophy (LVH), but the prognosis varies. Nevertheless, the feasibility of distinguishing these two conditions is limited by the fact that overlapping LVH and diverse forms of HCM can often lead to diagnostic challenge when diagnosis is based on a single morphological index. Diagnosis is more difficult in a patient with a history of hypertension and with left ventricular wall thickness between 11 and 15 mm, (see Figures 1 & 2).
Meng Jiang and Lianming Wu et al. [1] have deduced an integrated formula based on cardiac magnetic resonance (CMR) imaging and established a differentiating flow-chart between HCM and hypertensive heart disease (HHD). In this study, the investigators aim to explore the applicability of the quantifying scheme for distinguishing HCM from HHD or recently known hypertensive cardiomyopathy (HTN-CM) in the multi-center trial [1].
The hypertrophic cardiomyopathy was diagnosed by left ventricular hypertrophy via echocardiography (wall thickness >15 mm) with either genetic determination of a pathogenic mutation or left ventricular hypertrophy (LVH) (end-diastolic wall thickness >15 mm) with resting left ventricular outflow tract obstruction or hypertrophy in a recognizable pattern, i.e., ventricular bulge in apical-variant HCM. In addition, then patients with hypertrophic cardiomyopathy were evaluated by the predetermined differentiating formula. On the other hand, the diagnosis of hypertensive heart disease was based on medical history and conventional echocardiography. Long durations of uncontrolled hypertension for at least 5 years with systolic blood pressure [BP] ≥140 mm Hg or diastolic BP ≥90 mm Hg or both in the absence of other cardiac or systemic diseases were used as criteria. In addition, then patients with hypertensive heart disease were evaluated by the predetermined differentiating formula Jiang et al. [1].
Diagnostic Test: evaluated by the predetermined differentiating formula
After recruiting patients, collecting the baseline data, a CMR scan carried out and post-processed, a predetermined differentiating formula (including left ventricular morphology, ejection fraction, presence of late gadolinium enhancement, T1 value and strain data) will be used to produce a cardiac values , which is to be input into differentiating flow.
Criteria
Hypertrophic cardiomyopathy: Genetic determination of a pathogenic mutation or LVH (end-diastolic wall thickness >15 mm) with resting left ventricular outflow tract obstruction or hypertrophy in a recognizable pattern, i.e., ventricular bulge in apical-variant HCM. Of note, patients with documented HCM were divided into subgroups based on whether concomitant with hypertension or left ventricular outflow tract (LVOT) obstruction.s
Hypertensive Heart Disease: Diagnosis depends on history of long durations of uncontrolled hypertension (systolic blood pressure≥140 mm Hg or diastolic blood pressure ≥90 mm Hg); Echocardiography: left ventricular wall thickness in diastolic >11mm; Absence of other cardiac or systemic diseases; left ventricular mass/body surface area >81 g/m2 (Male) or >61 g/ m2 (Female).
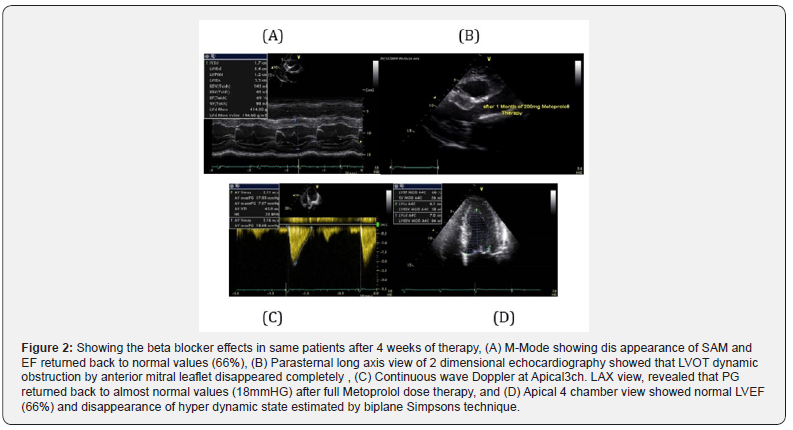
After 4 weeks of Full dose of Beta blockers therapy Metoprolol 200mg daily, the patient become a symptomatic and PG at LVOT returned back to normal values, see (Figure 2).
Overview in Hypertensive Cardiomyopathy
Hypertensive cardiomyopathy (HTN-CM) is a structural cardiac disorder generally accompanied by concentric left ventricular hypertrophy (LVH) associated with diastolic or systolic dysfunction in patients with persistent systemic hypertension. HTN-CM is difficult to distinguish from other cardiac diseases that cause myocardial hypertrophy, such as hypertrophic cardiomyopathy, Fabry disease, or cardiac amyloidosis. However, when other causes are ruled out, leaving hypertension the only possible cause for LVH development, this is considered to be HTNCM [2].
Hypertension (HTN) is a major global health issue, accounting for approximately 50% cases of both stroke and ischemic heart disease, and approximately 13% of the total deaths worldwide [2]. Persistent hypertension can cause structural and functional myocardial abnormalities such as LVH and remodeling, which are frequently seen in patients with hypertension [3].
Importantly, the Framingham Heart Study revealed that LVH is a risk factor for cardiovascular morbidity and mortality, independent of other cardiovascular risk factors, including elevated blood pressure itself [4,5]. In addition, patients with persistent hypertension and LVH are susceptible to sudden death [6].
In this review article, we summarize the diagnostic evaluation and differentiation of LVH and management options of HTN-CM on clinical background. We have focused on human studies in order to emphasize the importance of early identification and optimization of treatment in patients with hypertension and cardiac hypertrophy
Epidemiology
The prevalence of LVH varies with the severity of hypertension, ranging from 20% in mild to almost 100% in severe or complicated hypertension [6].
LVH in patients with hypertension predominantly results not only from a chronic increase in LV afterload but also a genetic component such as the DD genotype of the Angiotensin- Converting-Enzyme (ACE) gene and B2 bradykinin receptor polymorphism [7-9].
Furthermore, the prevalence of LVH among hypertensive patients is influenced by gender, obesity, and possibly age. Sexspecific criteria for LV mass index identify LVH in more women than men with systemic hypertension [10].
Myocardial Remodeling and Patterns of LVH in HTN-CM in Comparison to HCM
Conventional echocardiography provides useful morphological information of LVH patterns. For example, patients with hypertrophic cardiomyopathy (HCM) frequently show asymmetrical septal hypertrophy of the LV; this is the most characteristic finding [11]. In contrast, LVH associated with hypertension or HTN-CM is characterized by symmetrical (concentric) LVH. However, 13%-31% of patients with HCM show symmetrical hypertrophy [12], whereas 4%-47% of hypertensive patients manifest asymmetrical septal hypertrophy [13].
Velagaleti recently reported that the data from the Framingham Heart Study revealed that heart failure risk varied by LV geometric pattern, with eccentric and concentric hypertrophy predisposing to heart failure with reduced and preserved ejection fraction, respectively, after a mean follow-up of 21 years [14].
Clinical Manifestation in Patients With HTN-CM: Case Study
See (Figures 1 and 2) A thorough history and physical should be performed. All patients should be asked about family history of HOCM and of sudden cardiac death. A majority of hypertensive and HCM patients are asymptomatic. Dyspnea is the most common complaint among symptomatic patients. Patients may also complain of pre syncope, syncope, angina, palpitations (secondary to arrhythmia), or dizziness. Symptoms will frequently be exacerbated by exertion. Severe cases will present like congestive heart failure with paroxysmal nocturnal dyspnea, leg edema, and orthopnea. The most devastating presentation is sudden cardiac death [15].
Physical Exam will not provide a definitive diagnosis, but it should provide clues to increase clinical suspicion. Patients may present with a jugular venous pulse with prominent A wave, S4 heart sound, split second heart sound (may paradoxically split in severe outflow tract obstruction), double carotid pulse, systolic ejection crescendo-decrescendo murmur, laterally displaced apical precordial impulse that may be abnormal forceful or enlarged, and/or a pan systolic murmur at the apex and axilla of mitral regurgitation with systolic anterior wall motion. Lung exam should be normal [15].
Certain maneuvers can affect murmurs auscultated in HOCM and HTN-CM with LVOT dynamic obstruction; hand grip will increase ventricular volume decreasing LV outflow gradient and decrease murmur intensity and valsalva maneuver which decrease ventricular volume increasing LV outflow gradient and increase murmur intensity [15].
Persistent systemic hypertension induces LVH, fibrosis, diastolic dysfunction, and an increase in the activation of the renin angiotensin aldosterone system (RAAS), which leads to congestive heart failure [16]. One of the mechanisms of heart failure in patients with hypertension is LV diastolic dysfunction. LV diastolic dysfunction associated with hypertension is morphologically characterized by LV wall thickening and increased Left Atrial (LA) volume. In advanced stages, hypertension induces eccentric LVH and LV systolic dysfunction, see Figure (1) [17].
Role of Cardiac Imaging in Diagnosis of HTN-CM
Echocardiography and Speckle Tracking Echocardiography, see Figure (3):
Echocardiography is a powerful tool that provides morphological information about the LVH pattern in patients with hypertension. LVH can be detected with both electrocardiography (ECG) and echocardiography, although echocardiography is more sensitive and accurate in LVH detection than ECG [18].
Assessment of diastolic dysfunction by echocardiography is also important in the management of patients with HTN-CM. Diastolic dysfunction (DD) is seen in approximately 50% of patients with hypertension [19]. The changes in conventional Doppler echocardiographic parameters, such as peak early filling velocity (E), late diastolic filling velocity (A) and its ratio, as well as deceleration time, should be monitored. Patients with longstanding hypertension and advanced stage of HTN-CM may show a pseudo normalization of E/A ratio (Stage II Moderate DD) and restrictive filling physiology pattern (stage III-IV severe DD).
Tissue Doppler Imaging (TDI) allows quantitative assessment of ventricular function and early diastolic mitral annular velocity (E′); the ratio of E/E′, which is a parameter with correction of preload. This is a useful tool to assess the severity of diastolic dysfunction and elevated filling pressure of left ventricle and LA pressure in patients with HTN-CM [19]. Kasner et al. [20] performed both invasive and noninvasive assessment of diastolic dysfunction and identified the LV filling index of E/E′ (lateral) as the best index for detection of diastolic dysfunction in patients with heart failure with normal ejection fraction.
Strain and strain rate parameters derived from TDI, as well as speckle tracking echocardiography have also been reported as useful tools for detection of diastolic dysfunction, and these can aid in discriminating patients with HTN-CM from those with other causes of LVH [21]. The abnormalities in strain parameters may occur in a stage of subclinical diastolic dysfunction as well as systolic dysfunction in hypertensive patients [22,23] making this a useful strategy for disease prevention and prognosis of high risk patients susceptible for heart failure and acute pulmonary edema, see Figure 3, [24].
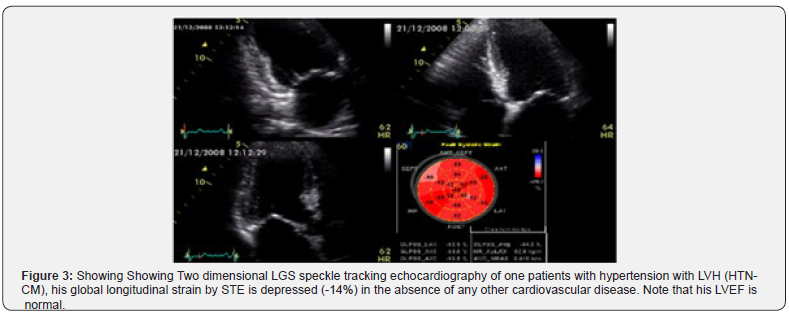
The early clinical abnormalities in global longitudinal systolic (GLS) strain STE (systolic dysfunction) and diminished E annular velocity (diastolic dysfunction) with Tissue Doppler study (TDE) can be demonstrated in apparently a symptomatic hypertensive patient [24,25]. Diabetes and hypertension, which are often associated with the impairment of GLS, are also common findings in patients with heart failure with preserved ejection fraction (HFpEF), and it is well known that LVH is the key structural change of this disease. Probably, repetitive ischemic insults due to macro vascular and micro vascular abnormalities and interstitial fibrosis cause an early intrinsic depression of subendocardial longitudinal fiber contractility in these patients, especially in more hypertrophic hearts. For this reason, longitudinal myocardial performance is early impaired, whereas in the first stage of the disease the sparing of circumferential fibers results in EF remaining within the normal range [26]. So, GLS provides additional information in the evaluation of LVH, and regional changes in longitudinal strain seem to identify specific myocardial deformation patterns for some forms of myocardial hypertrophy [27]. It is clear nowadays that myocardial fiber mechanical dysfunction precedes histopathological changes such as fibrosis and hypertrophy in essential hypertension which can be detected in the preclinical stage of the disease, through a reduced tissue Doppler –derived systolic and diastolic velocities and depressed GLS either before or even without the development of LVH [24- 27].
Furthermore, Thor Edvardsen and his colleagues revealed an early systolic dysfunction criteria of the LV evidenced by STE in heart failure with preserved ejection fraction (HFpEF) [28].
Generally, global longitudinal systolic strain STE and TDI are of crucial importance in the subclinical diagnosis of myocardial diseases specifically in HCM (+) genotype, diabetes- induced cardiomyopathy and in hypertensive heart disease.
In summary, myocardial strain is an important clinical tool for the quantification of Left Ventricular (LV) function which is now feasible with STE. The best evaluated strain parameter is Global Longitudinal Strain (GLS) which is more sensitive than LVEF as a measure of systolic function and can be used to identify sub-clinical LV dysfunction in different forms of hypertrophic cardiomyopathies.
Cardiac magnetic resonance imaging (CRM)
CMR offers a unique opportunity for noninvasive quantitation of both LVH with high reproducibility and myocardial fibrosis with high spatial and contrast resolution [29].
CRM can identify the pathophysiology of hypertension disease process and provides accurate and reproducible measures of ventricular volumes, mass, function, geometry of LV and hemodynamics as well as uniquely allowing tissue characterization of diffuse and focal fibrosis [30]. CRM can detect as well an increased type I pro collagen synthesis by Late Gadolinium Myocardial Enhancement (LGE). In addition, CRM has been proposed as preclinical marker of those patients with cardiac hypertrophy at risk of ventricular arrhythmias and SCD [31].
Advances in CMR have made it possible to evaluate both the structure and function of the heart. Extensive LGE by CMR can identify high-risk patients with LVH and HCM as well. This is an independent predictor of SCD, a quantitative assessment of myocardial fibrosis by LGE and as such, it can thus be a clinically useful tool in order to help risk stratify patients with cardiac hypertrophy whether he/she has inherited disease as hypertrophic obstructive cardiomyopathy (HOCM), idiopathic LVH or acquired disease (hypertensive heart disease) [32-33].
In summary, CRM is the diagnostic imaging technique of choice to differentiate HCM (cardiac myocite disarray) from HTNCM noninvasively and can be able to define susceptible patients at risk of SCD and major cardiac arrhythmias.
Treatment of HTN-CM
Hypertensive cardiomyopathy (HTN-CM) is a result of a complex interaction of genetic and hemodynamic factors inducing structural and functional adaptations [34]. LVH in HTN-CM is a recognized risk factor for congestive heart failure, dysrhythmia, and sudden death [35]. Better elucidation of the mechanisms producing cardiovascular abnormalities should lead to treatment targeted at reducing the effects of hypertension on the heart and vascular system. Most antihypertensive treatments promote regression of LVH and reversal of diastolic dysfunction, which may decrease symptoms of congestive heart failure and improve survival rates [36].
The RAAS is implicated in the development of cardiac hypertrophy associated with pressure overload [37]. Brilla et al. [38] indicated ACE inhibition with lisinopril can regress myocardial fibrosis, regardless of LVH regression, and is accompanied by improved LV diastolic function. The Losartan Intervention for Endpoint Reduction (LIFE) study showed that the angiotensin II type 1 (AT1) receptor antagonist (ARBS), Losartan, reduced LV mass and improved systolic performance, despite only a small drop in blood pressure [39].
Although ACE inhibitors and ARBS are effective therapies in HTN, HTN-CM and heart failure. It should not be prescribed for those patients who have dynamic LVOT obstruction. Alternatively, beta blockers are drug of choice in such patients.
Interestingly, Chang et al. [40] showed that Rosuvastatin therapy attenuated myocardial fibrosis and LV stiffness.
The most important issues in the treatment of HTN-CM are appropriate blood pressure control, weight loss, and dietary sodium restriction. Regression of LVH and, more importantly, the prognosis of patients with HTN-CM, are both highly related to the antihypertensive response as well as the therapy used [41].
Importantly, negative inotropic therapy of beta blockers represent a beneficial therapeutic approach in selected patients with dynamic left ventricular outflow tract obstruction in HHD with LVH [42]. Similarly, in population-based HOCM cohorts heart failure is a dominant cause of death and on multivariate analysis beta-blocker therapy was associated with a dose-dependent cardioprotective effect on total, disease-related as well as heart failure-related mortality [43].
Key points
Chronic blood pressure elevation induces a number of cardiac adaptive and detrimental changes in three main territories: left ventricle, coronary arteries, and left atrium. Consequently, a comprehensive definition of hypertensive heart disease should include the most important alterations documented in these three territories. The VIA classification includes cardiac involvement secondary to chronic hypertension in the three main territories ranked by increasing degree of severity: V (left ventricle) (0: none; 1: hypertrophy; 2: diastolic dysfunction; 3: systolic dysfunction); I (myocardial ischaemia) (0: none; 1: microvascular ischaemia; 2: macrovascular ischaemia; 3: myocardial infarction); A (atrial fibrillation) (0: none; 1: paroxysmal; 2: permanent; 3: embolic).
Conclusion
HTN-CM is characterized by LVH and LVH-induced diastolic dysfunction more frequently than systolic dysfunction. This is associated with increased risk of heart failure, arrhythmias, and death. LVH itself is a risk factor for mortality and morbidity, independent of other cardiovascular risk factors, including high blood pressure. Therefore, early detection of LVH development in patients with hypertension is important in order to start effective treatment when the myocardial damage is still reversible. Echocardiography is the best screening and an ideal tool for detection of LVH and LV myocardial dysfunction in its early stage, along with advanced measurements such as TDE and strain parameters (STE). CMR represents another powerful tool for detection and discrimination of patients with HTN-CM from those with other LVH diseases (HCM, amyloidosis and Fabrys disease). Because the regression of LVH is associated with a reduction in cardiovascular risk and improved cardiac function, achieving good blood pressure control is very important in the treatment of patients with HTN-CM. This can be achieved with the use of antihypertensive agents (ACE inhibitors, ARBS, and aldosterone receptor antagonists or even calcium antagonists), which can be effective for reverse remodeling of the myocardium, unless LVOT dynamic obstruction is present.
Beta Blockers are the best option in management of HTN-CM in the presence of significant LVOT obstruction as well as in HOCM.
These observations emphasize the importance of early diagnosis and effective treatment of hypertensive heart disease with LVH (HTN-CM) to prevent cardiac complications.
References
- Meng Jiang and Lianming Wu (2017) Differentiating Hypertrophic Cardiomyopathy From Hypertensive Heart Disease. NIH U.S. National Library of Medicine. Clinical Trials Gov NCT03271385.
- Kishio Kuroda, Tomoko S Kato, Atsushi Amano (2015) Hypertensive cardiomyopathy: A clinical approach and literature review. World J Hypertens 5(2): 41-52.
- Nadruz W (2015) Myocardial remodeling in hypertension. J Hum Hypertens 29(1): 1-6.
- Janardhanan R, Kramer CM (2011) Imaging in hypertensive heart disease. Expert Rev Cardiovasc Ther 9(2): 199-209.
- Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP (1990) Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med 322(22): 1561-1566.
- Ruilope LM, Schmieder RE (2008) Left ventricular hypertrophy and clinical outcomes in hypertensive patients. Am J Hypertens 21(5): 500-508.
- Ohishi M, Rakugi H, Ogihara T (1994) Association between a deletion polymorphism of the angiotensin-converting-enzyme gene and left ventricular hypertrophy. N Engl J Med 331(16): 1097-1098.
- Brull D, Dhamrait S, Myerson S, Erdmann J, Woods D, et al. (2001) Bradykinin B2BKR receptor polymorphism and left-ventricular growth response. Lancet 358(9288): 1155-1156.
- Kizer JR, Arnett DK, Bella JN, Paranicas M, Rao DC, et al. (2004) DC Differences in left ventricular structure between black and white hypertensive adults: the Hypertension Genetic Epidemiology Network study. Hypertension 43(6): 1182-1188.
- Ganau A, Devereux RB, Roman MJ, de Simone G, Pickering TG, et al. (1992) Patterns of left ventricular hypertrophy and geometric remodeling in essential hypertension. J Am Coll Cardiol 19(7): 1550-1558.
- Wigle ED, Rakowski H, Kimball BP, Williams WG (1995) Hypertrophic cardiomyopathy. Clinical spectrum and treatment. Circulation 92(7): 1680-1692.
- Shapiro LM, McKenna WJ (1983) Distribution of left ventricular hypertrophy in hypertrophic cardiomyopathy: a two-dimensional echocardiographic study. J Am Coll Cardiol 2(3): 437-444.
- Dunn FG, Chandraratna P, deCarvalho JG, Basta LL, Frohlich ED (1997) Pathophysiologic assessment of hypertensive heart disease with echocardiography. Am J Cardiol 39(6): 789-795.
- Velagaleti RS, Gona P, Pencina MJ, Aragam J, Wang TJ, et al. (2014) Left ventricular hypertrophy patterns and incidence of heart failure with preserved versus reduced ejection fraction. Am J Cardiol 113(1): 117-122.
- Marc A. Raj, Amandeep Goyal (2019) Hypertrophic Obstructive Cardiomyopathy. StatPearls Publishing LLC. Bookshelf ID: NBK430820.
- Gonzalez A, Lopez B, Diez J (2004) Fibrosis in hypertensive heart disease: role of the renin-angiotensin-aldosterone system. Med Clin North Am 88(1): 83-97.
- Devereux RB, Bella JN, Palmieri V, Oberman A, Kitzman DW, et al. (2001) Left ventricular systolic dysfunction in a biracial sample of hypertensive adults: The Hypertension Genetic Epidemiology Network (HyperGEN) Study. Hypertension 38(3): 417-423.
- Lorell BH, Carabello BA (2000) Left ventricular hypertrophy: pathogenesis, detection, and prognosis. Circulation 102(4): 470-479.
- Kasner M, Westermann D, Steendijk P, Gaub R, Wilkenshoff U, et al. (2007) Utility of Doppler echocardiography and tissue Doppler imaging in the estimation of diastolic function in heart failure with normal ejection fraction: a comparative Doppler-conductance catheterization study. Circulation 116(6): 637-47.
- Kato TS, Izawa H, Komamura K, Noda A, Asano H, et al. (2008) Heterogeneity of regional systolic function detected by tissue Doppler imaging is linked to impaired global left ventricular relaxation in hypertrophic cardiomyopathy. Heart 94(10): 1302-1306.
- Narayanan A, Aurigemma GP, Chinali M, Hill JC, Meyer TE et al. (2009) Cardiac mechanics in mild hypertensive heart disease: a speckle-strain imaging study. Circ Cardiova Imaging 2(5): 382-390.
- Solomon SD, Janardhanan R, Verma A, Bourgoun M, Daley WL, et al. (2007) Effect of angiotensin receptor blockade and antihypertensive drugs on diastolic function in patients with hypertension and diastolic dysfunction: a randomised trial. Lancet. 369(9579): 2079-2087.
- Rudolph A, Abdel-Aty H, Bohl S, Boye P, Zagrosek A, et al. (2009) Noninvasive detection of fibrosis applying contrast-enhanced cardiac magnetic resonance in different forms of left ventricular hypertrophy relation to remodeling. J Am Coll Cardiol 53(3): 284-291.
- Nagib Elkilany GE (2008) The Crucial Role of Global Strain in Identifying Patients with Subtle Systolic Dysfunction and Patients at Risk for Acute Pulmonary Edema, Euro Echo, Spain.
- Galal Eldin Nagib Elkilany, Maryam De Groef, Ibrahim Kabbash (2011) How to Identify Latent Systolic Dysfunction and Post-Operative Risk in Patients with Mitral Incompetence and Normal Ejection Fraction. World Journal of Cardiovascular Surgery 1(2): 11-17.
- Todaro MC, Khandheria BK, Longobardo L, Zito C, Cusmà-Piccione M, et al. (2015) New diagnostic perspectives on heart failure with preserved ejection fraction: Systolic function beyond ejection fraction. J Cardiovasc Med (Hagerstown) 16(8): 527-537.
- Sushil A, Luis, Jonathan Chan, Patricia A, Pellikka (2019) Echocardiographic Assessment of Left Ventricular Systolic Function: An Overview of Contemporary Techniques, Including Speckle-Tracking Echocardiography. 2018 Mayo Foundation for Medical Education and Research n Mayo Clin Proc 94(1): 125-138.
- Thor Edvardsen, Thomas Helle Valle, Otto A, Smiseth (2006) Systolic Dysfunction in Heart Failure with Normal Ejection Fraction: Speckle-Tracking Echocardiography. Prog Cardiovasc Dis 49(3): 207-214.
- Rudolph A, Abdel Aty H, Bohl S, Boyé P, Zagrosek A, et al. (2009) Noninvasive detection of fibrosis applying contrast-enhanced cardiac magnetic resonance in different forms of left ventricular hypertrophy relation to remodeling. J Am Coll Cardiol 53(3): 284-291.
- Alicia M Maceira, Raad H Mohiaddin (2012) Cardiovascular magnetic resonance in systemic hypertension. Journal of Cardiovascular Magnetic Resonance 14: 28.
- Weng Z, Yao J, Chan RH, He J, Yang X, et al. (2016) Prognostic Value of LGE-CMR in HCM A Meta-Analysis, JACC: 9(12): 1392-1402.
- Green JJ, Berger JS, Kramer CM, Salerno M (2012) Prognostic value of late gadolinium enhancement in clinical outcomes for hypertrophic cardiomyopathy. J Am Coll Cardiol 5(4): 370-377.
- Diamond JA, Phillips RA (2003) Regression of left ventricular hypertrophy: are there preferred drugs? Curr Hypertens Rep 5(5): 368-371.
- Verdecchia P, Schillaci G, Borgioni C, Ciucci A, Gattobigio R, et al. (1996) Prognostic value of left ventricular mass and geometry in systemic hypertension with left ventricular hypertrophy. Am J Cardiol 78(2): 197-202.
- Diamond JA, Phillips RA (2003) Regression of left ventricular hypertrophy: are there preferred drugs? Curr Hypertens Rep 5(5): 368-371.
- Dzau VJ (1993) Tissue renin-angiotensin system in myocardial hypertrophy and failure. Arch Intern Med 153(8): 937-942.
- Brilla CG, Funck RC, Rupp H (2000) Lisinopril-mediated regression of myocardial fibrosis in patients with hypertensive heart disease. Circulation 102: 1388-1393.
- Bang CN, Gerdts E, Aurigemma GP, Boman K, Dahlöf B, et al. (2013) Systolic left ventricular function according to left ventricular concentricity and dilatation in hypertensive patients: the Losartan Intervention For Endpoint reduction in hypertension study. J Hypertens 31(10): 2060-2068.
- Chang SA, Kim YJ, Lee HW, Kim DH, Kim HK, et al. (2009) Effect of rosuvastatin on cardiac remodeling, function, and progression to heart failure in hypertensive heart with established left ventricular hypertrophy. Hypertension 54(3): 591-597.
- Ruilope LM, Schmieder RE (2008) Left ventricular hypertrophy and clinical outcomes in hypertensive patients. Am J Hypertens 21(5): 500-508.
- Cabrera Bueno F, García Pinilla JM, Gómez Doblas JJ, Montiel Trujillo A, Rodríguez Bailón I, et al. (2006) Beta-blocker therapy for dynamic left ventricular outflow tract obstruction induced by exercise. Int J Cardiol 117(2): 222-226.
- Javidgonbadi D, Andersson B, Abdon NJ, Schaufelberger M, Östman-Smith I (2019) Factors influencing long-term heart failure mortality in patients with obstructive hypertrophic cardiomyopathy in Western Sweden: probable dose-related protection from beta-blocker therapy. Open Heart 6(1): e000963.
- Gonzalez Maqueda, Eduardo Alegria Ezquerra, Jose Ramon Gonzalez Juanatey (2009) Hypertensive heart disease: a new clinical classification (VIA). An article from the E-Journal of the ESC Council for Cardiology Practice 7(20): 12.






























