Arrythmia in Congenital Heart Diseases, Where Are We?
Amira Nour*, Mostafa Abdelmonaem, Mohamed Mousa, Alaa Roushdy and Heba Kamel
Department of Cardiology, Ain Shams University Hospital, Egypt
Submission: November 08, 2019; Published: December 17, 2019
*Corresponding author: Amira Nour, Congenital and Structural Heart Disease Unit, Department of Cardiology, Ain Shams University Hospital, Abbassya, Cairo, Egypt
How to cite this article:Amira Nour, Mostafa Abdelmonaem, Mohamed Mousa, Alaa Roushdy, Heba Kamel. Arrythmia in Congenital Heart Diseases, Where Are We?. J Cardiol & Cardiovasc Ther. 2019; 15(3): 555914. DOI: 10.19080/JOCCT.2019.15.555914
Abstract
Background: Arrythmia is a major cause of cardiovascular morbidity and mortality in patients with congenital heart diseases. It has multifactorial predisposing factor as congenitally malformed or displaced conduction system, hemodynamic, mechanical or hypoxic stress and residual or postoperative sequelae.
Methods: This was a prospective observational study involving all patients who were following up or referred to congenital and structural heart disease unit in our department over a period of 42 months and were discovered to have significant symptomatic cardiac arrhythmia. A detailed custom-made sheet was applied to all eligible patients to collect demographic data, underlying congenital heart disease, type of arrhythmia and possible management and/or outcome.
Results: The study population included 107 patients with different congenital heart diseases with mean age 20.47+/- 13.2. Bradyarrhythmia were more common (67%) than tachyarrhythmia. Post-operative CHB was the most common bradyarrythmia. One third of the patients had atrial or ventricular tachyarrythmia. Post Fallot repair patients developed ventricular tachycardia while patients with Ebstein anomaly more commonly developed atrial tachycardias, flutter or SVT. Patients with CHB were managed with transvenous pacing (51%), epicardial pacing (16%), two patients with CCTGA with impaired RV functions underwent CRT implantation, two patients post Fallot repair underwent ICD implantation.
Conclusion: Patients with CHD are subjected to wide range of arrhythmias. Postoperative complete heart block was the commonest arrhythmia in this registry especially in patients with L-TGA, AVSD and VSD. Tachyarrythmias accounted for one third of the patients. Atriotomy incisions, sutures, and conduit is a major risk factor for post-operative atrial tachycardia. The use of ICDs and CRT in patient with congenital heart diseases have proven relative efficacy.
Keywords: Congenital heart disease; Atrial arrythmia; Ventricular arrythmia; ICD; CRT
Abbreviations: CHD: Congenital Heart Diseases; PACs: Premature Atrial Contractions; PVCs: Premature Ventricular Contractions; ICD: Implantable Cardiac Defibrillator, CRT: Cardiac Resynchronization Therapy; Standard Deviation; CHB: Complete Heart Block; AVRT: Atrioventricular Tachycardia JET: Junctional Ectopic Tachycardia
Introduction
Congenital heart diseases (CHD) represent 1% of the annual livebirths; arrythmia is a common cause of hospital admission and late mortality in this group of patients [1]. Due to the recent advances in the surgical and interventional cardiovascular management of CHD, the population of the adult patients with CHD exceeded markedly the number of the children [2]. As consequence of these operative repairs and the added longevity, arrythmias are becoming increasingly common [3]. Moreover, arrythmias are poorly tolerated in those with CHD leading to more hemodynamic compromise and worse cardiovascular outcome [4].
Arrythmia in congenital heart disease has different etiologies, it may be due to inherent congenital anomaly, however postoperative arrythmia accounts for the majority of these arrythmia [5]. The underlying postoperative mechanisms are multifactorial may be due to direct injury or manipulation to the conduction system, local tissue edema and inflammation to the myocardial tissue adjacent to the conductive system [6]. Other considerable risk factors are the postoperative inotropes and vasopressors which are highly arrythmogenic, and the electrolyte imbalances frequently seen after major cardiac surgeries contributing to the long cross clamping time and cardiac bypass machine time [7]. Despite the fact that these postoperative arrythmia are commonly encountered early postoperative, they are usually transient and recover with proper management [8].
Our Ain shams congenital heart disease center for both pediatric and adult population continue to be at the forefront for multidisciplinary management of these patient with complex cardiac anatomy and who require multidisciplinary management strategies including antiarrhythmic therapy, specific electrophysiological studies, device therapy and catheter ablation. The current study represents a prospective registery of different types of arrhythmias encountered in these patients as well as their management and outcome.
Methods
This study was approved by our institutional review board and informed consent was applied from the enrolled patients or their guardians.
This was a prospective observational study done in Ain Shams university hospitals, which a tertiary referral center with a congenital and structural heart disease unit. The study population consists of consecutive population of all congenital heart disease patients who presented to Ain Shams university hospitals, the structural and congenital heart disease unit for medical treatment , catheter or surgical interventions or were following up in the outpatient clinic and suffered from significant symptomatic cardiac arrythmia from January 1, 2016 till June 30, 2019.
A detailed custom-made sheet was applied to all eligible patients to collect demographic data, underlying congenital heart disease, type of arrhythmia and possible management and/or outcome. All types of significant symptomatic arrythmia were recorded using a 3channel standard 12-lead electrocardiogram recorder or 24 or 48 hours Holter when needed. Arrythmias which were identified by the primary cardiac team were confirmed by the electrophysiologist.
Arrythmia was defined as an abnormal rhythm that was present for over 30 seconds anytime during the inpatient or postoperative course. Premature atrial contractions (PACs) or premature ventricular contractions (PVCs) were not included if they were infrequent (< 10 per minute). Different types of management were included as electrical or pharmacological cardioversion, electrical cardiac pacing, implantable cardiac defibrillator (ICD), cardiac resynchronization therapy (CRT), overdrive suppression, transcutanous ablation or pharmacological treatment.
Statistical Analysis
Statistical analysis was performed using SPSS (statistical package version sixteen), Categorical variables were expressed as absolute and relative frequencies (percentage) while continuous variables were presented as mean values ± standard deviation (SD). Comparisons were made between the two groups using t-test for continuous variables and chi-square test and Pearson correlation coefficient for categorical variables, Difference was considered statistically significant at a P value < 0.05 and highly significant at P value < 0.01.
Results
The study population included 107 patients with different congenital heart diseases, there were 57 males (53%) and 50 females (47%) with mean age 20.47+/- 13.2 with 3 patients ≤ 6 months. Bradyarrhythmia including complete heart block (CHB) and second degree heart block (2nd HB) were more common (67 %) than tachyarrhythmia. CHB accounted for the most common arrythmia (66.4%) in the study population. The incidence of cardiac arrythmia is presented in Table 1.
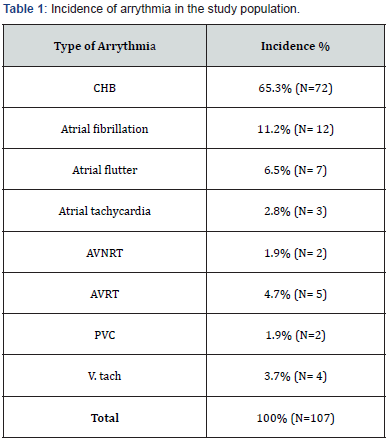
The most common CHD was ASD (n=31, 29 %), followed by VSD and AVSD (n= 19, 17.7 %). most of these patients developed postoperative CHB, however two patients in our study developed transient CHB after transcatheter ASD closure and 1 patient developed permanent CHB after transcatheter closure of subaortic peri membranous VSD.
Symptomatic bradyarrythmia was also significant in patients with congenitally corrected transposition of the great vessels (L-TGA) (15.8 %), those with complete transposition of the great vessels after Senning or Mustrad operation (1.8%) and those who underwent TV repair (5.6 %) (Table 2).
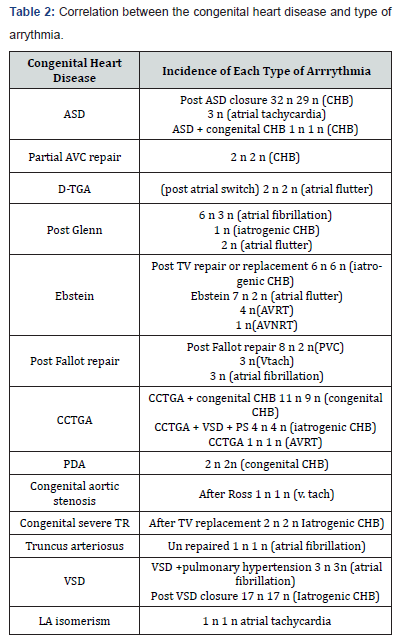
On the other hand tachyarrythmia accounted for 33 % of the total population, almost one quarter of them were post fallot repair patients who developed either atrial or ventricular tachycardia, similarly Ebstein anomaly accounted for 6.5 % of the patients who either developed atrial tachycardia, atrial flutter or SVT.
The most common arrythmia in children (< 16 years) was postoperative CHB (38%), and most common arrythmia in adulthood was also CHB (28 %) mostly associated with CCTGA patients followed by atrial fibrillation mostly in patients with ASD with late presentation older than 25 years.
Postoperative arrythmia was the most common etiology of arrythmia in the study population (65.4 %), permanent CHB necessitating pacemaker therapy was frequently seen followed by atrial tachycardias then atrial flutter. Risk factors which increase the incidence of postoperative arrythmia was related to younger age, type of cardiac surgery, long cross clamp time, prolonged cardiopulmonary bypass time, use of more than one positive inotropic drug
In our study arrhythmia was associated with pregnancy in 5 patients, two of them with CCTGA developed CHB during pregnancy, two patient with Ebstein anomaly who developed atrial flutter and atrioventricular tachycardia (AVRT) and one patient with single ventricle post Glenn developed atrial fibrillation. Factors increasing incidence of recurrence of arrythmia was found to be patient with complex congenital heart disease, history of previous cardiac surgery, oxygen saturation, pregnancy.
Most of the patients with CHB were managed with transvenous pacing (51%), few patients managed with epicardial pacing (16%) , most patients with atrial fibrillation or atrial flutter were managed conservatively with cardioversion and medical treatment (16.8%), two patients with CCTGA with impaired RV functions underwent CRT implantation, two patients post Fallot repair underwent ICD implantation.
Regarding the cardiovascular outcome, a total of 92 patients improved, 7 patients underwent successful ablation for AVRT and AVNRT, 11 patients with atrial fibrillation and atrial flutter underwent successful electrical cardioversion, 2 patients with CCTGA were responders to CRT implantation, 1 post Fallot repair with successful ICD implantation, 49 patients with transvenous PM either DDD, VVI, VDD or in some cases with complex congenital heart disease the lead was implanted in the coronary sinus, one patient underwent simultaneous ASD transcatheter closure and DDD implantation in one setting.
Eleven patients developed recurrence post ablation; three patients with recurrence of atrial flutter after successful ablation, 5 patients with atrial fibrillation and complex congenital heart disease, 3 patients with Ebstein anomaly with recurrence of AVRT, one patient died post ICD implantation with malignant ventricular tachycardia.
Discussion
Atrial and ventricular arrythmias are a major cause of cardiovascular morbidity and mortality in patients with CHD [9,10]. Unfortunately most of studies discussed certain type of arrythmia and its management [10] or discussed only postoperative arrythmia in pediatric patients with CHD [11], there is a relative paucity in literature for guidelines on management of arrythmias in CHDs due to the diverse nature of CHDs from simple to complex lesions and the different types of arrythmias encountered in these patients [12].
In the current study we report the incidence and etiology of different types of arrythmia and the commonly associated defects. Similar to our study, Edeward P walsh and his coworkers reported that atrial tachycardia and supraventricular tachycardia were commonly seen in Ebstein anomaly patients, ventricular tachycardia mostly seen in post Fallot repair patients, atrial fibrillation mostly seen in patients with single ventricle physiology while congenital heart block was frequently reported in CCTGA patients, meanwhile post-operative CHB was seen in patients after VSD or AVSD closure [13].
The most common post-operative arrythmia was CHB more commonly transient due to edema and inflammation of the AV nodal conduction system , of note that 2.7 % of patients with CCTGA had CHB after VSD closure, 13.8 % of patient with VSD had postoperative CHB, 11 % of the patients with atrioventricular septal defect had postoperative permanent CHB , 4 % of the patients with ASD had permanent CHB. Moreover Khairy et al. [14] and his co-workers reported in their study that 7 % with AVSD had postoperative CHB, 4% of the patients with VSD had postoperative CHB, 25 % of the patients with CCTGA developed CHB after VSD surgical closure [14]. However, in contrast to ours, J.S Nelson reported that the most common post-operative arrythmia was the junctional ectopic tachycardia (JET) 6 % followed by CHB 2.5% [11]. A unique finding in our study is the recording of postoperative VT in a 5-year-old patient after Ross procedure mostly due to prolonged cross-clamp time and the use of more than one postoperative inotrope which was resistant to medical treatment and responded to lidocaine.
Pregnancy poses a great cardiovascular risk and significant hemodynamic comprise in patients with CHD especially those with complex cyanotic CHD [15]. In our study, 5 patients (4.7%) of the study group had arrhythmia during pregnancy; 3 pregnant ladies who had CCTGA developed CHB , two of them were presented for the first time during pregnancy, one of them was associated with impaired RV functions and underwent CRT implantation, the other two patients with severe TR had DDD implantation . one patient with severe form of Ebstein developed atrial flutter who underwent catheter ablation , and 1 patient with complex congenital heart disease post Glenn who developed atrial fibrillation was cardioverted successful, meanwhile Tateno and his coworkers reported that the incidence of arrythmias in pregnant women with CHD was 20% , including 4 patients with CCTGA who had CHB, and 15 patient with SVT [16].
The AV conduction system may be displaced if atrial and ventricular septa are mal-aligned, AV connections are discordant, or if the heart is univentricular. As a general rule of thumb, if the AV conduction system is displaced, it tends to be more susceptible to degeneration, placing patients at greater risk for AV block [17]. Regarding the therapeutic options for brady arrythmia, 41% of the patients had transient CHB which resolved after giving steroids and atropine mostly due to edema of the conduction system and 32% had permanent CHB which underwent successful either DDD or VVI implantation, 17% were epicardial and 83% were transvenous, it is noteworthy that we had successful transvenous PM implantation in a 6 year old boy weighing 24 kg.
Two patients with Ebstein anomaly and TV valvuloplasty developed CHB had VVI implantation with fixation of the Lead in the Coronary sinus due to small RV. Similar to our study Attenhofer fost et al. [18] reported 11 patients with Ebstein anomaly needed permanent pacing for CHB, two of them had transvenous VVI implanation with placement of the lead in the coronary sinus or the cardiac vein [18].
In our study two patients with L-TGA, CHB and impaired RV functions had successful CRT implantation while Moore JP et al. [19] reported successful transvenous CRT implantation in 18 patients with L- TGA and impaired RV functions [19].
A 5 year old patient with large Secundum ASD and CHB underwent simultaneous ASD closure and VVI implantation in one setting, which was not reported in literature except in a medical center in India by Raghu Cherukupalli [20].
In the current study two patients had transient CHB after transcatheter ASD closure which recovered spontaneously after the first 24 hours. Nonetheless, Nehgme reported a 2-year-old patient recovered from second degree Mobitz type II AV block after initial corticosteroid treatment [21,22]. Even though there was no conduction disturbance initial after device implantation, late-onset complete AV block requiring permanent pacemaker might still occur 4 years later [23] In the current study one patient had CHB 6 months post transcatheter VSD closure necessitating transvenous PM implantation.
The management of patients with tachyarrhythmias include pharmacological therapy, catheter ablation, ICD implantation and surgical interventions [24]. Pharmacological therapy is often not well tolerated in patients with CHD due to systemic ventricular dysfunction, sinus node disease, negative inotropic effect, hypoxic stress which increases the incidence of pro-arrythmia [25]. Due to the limited data on the dosage and toxicity of anti-arrhythmic drugs for the various age group with CHD , medical treatment was used only in 17% of patients, 12% of them developed atrial fibrillation and underwent successful cardioversion followed by anti-arrythmic drug (mostly amiodarone), 5% with atrial flutter and atrial tachycardia received betablockers most of them regained their sinus rhythm, and one patient who developed ventricular tachycardia and prolonged QT mostly due to amiodarone toxicity, responded dramatically to lidocaine.
Anatomical complexities and difficult vascular accesses pose a challenge to catheter based interventions in CHD patients. Ablation is specifically difficult too because of the hypoxia of the tissue and the hypertrophy which makes difficult to make a transmural scar [11]. With the advent of three-dimensional electroanatomic mapping and advances in catheter technology permitting larger and deeper lesions, transcatheter ablation has emerged as a promising alternative for many patients with tachyarrhythmias [26]. In our study 9.3% (10 patients), patients underwent successful conventional ablation. 8 patients with Ebstein anomaly and right sided accessory pathway underwent successful ablation with rate of recurrence reaching up to 25% the cases. Meanwhile Reich JD et al. [27] reported 65 patients with Ebstein anomaly and right sided accessory pathway with short term ablation success rate reaching up to 90% with late recurrence reaching up to 32% [27]. Mapping and ablation can be challenging as signals in the atrialized portion of the right ventricle may be complex and the true AV groove, along which accessory pathways are targeted, may not be readily apparent which increases the incidence of recurrence [28].
It is noteworthy to say that a 17-year-old patient with CCTGA had right sided accessory pathway underwent successful conventional ablation, another 14year old D-TGA patient post intra-atrial baffle repair (mustard) operation who underwent successful ablation of atrial arrythmia of the pulmonary venous atrium.
Sudden cardiac death is the most common cause of mortality in post Fallot repair patients. Monomorphic ventricular tachycardia occurs in 10 % of post Fallot repair patients due to reentry tachycardia [29]. Risk factors include QRS interval > 180 ms, presence of RVOT patch, older age at repair. In our study 3 post Fallot repair patients underwent ICD insertion two of them survived cardiac arrest, another patient with high risk criteria who utilized it for primary prevention who had non-sustained VT with QRS > 180 and RVOT transannular patch.
Conclusion
The last decade has witnessed major advances in our understanding of arrhythmia mechanisms and therapeutic option. CHB commonly occurs in patients with L-TGA, AVSD and VSD. Atriotomy incisions, sutures, and conduit may lead to development of scar-based macro-reentrant atrial circuit. The use of ICDs and CRT in patient with congenital heart diseases have proven relative efficacy.
Conflict of Interest
We still need more use of the 3D anatomical mapping and growing advances in the use of catheter technology (thermal, cryo and laser ablation) for treatment of many patients with complex anatomy and tachyarrythmia together with further analysis for risk factors of recurrence.
We need thorough risk stratification for patients with congenital heart disease presented with significant arrhythmia. Long term follow up with special attention to issues related to sudden death, ICDs, catheter ablation and surgical therapy are needed.
References
- Warnes CA, Liberthson R, Danielson GK, Dore A, Harris L, et al. (2001) Task force 1: the changing profile of congenital heart disease in adult life. J Am Coll Cardiol 37(5): 1170-1175.
- Attenhofer Jost CH, Connolly HM, Edwards WD, Hayes D, Warnes CA, et al. (2005) Ebstein’s anomaly: review of a multifaceted congenital cardiac condition. Swiss Med Wkly 135(19-20): 269-281.
- Oh JK, Holmes DR, Hayes DL, Porter CB, Danielson GK (1985) Cardiac arrhythmias in patients with surgical repair of Ebstein’s anomaly. J Am Coll Cardiol 6(6): 1351-1357.
- Kalman JK, Van Hare GF, Olgin JE, Saxon LA, Stark SI, et al. (1996) Ablation of “incisional” reentrant atrial tachycardia complicating surgery for congenital heart disease. Circulation 93(3): 502-512.
- Delaney JW, Moltedo JM, Dziura JD, Kopf GS, Snyder CS (2006) Early postoperative arrhythmias after pediatric cardiac surgery. J Thorac Cardiovasc Surg 131(6): 1296-1300.
- Anselmi A, Abbate A, Girola F, Nasso G, Biondi-Zoccai GG, et al. (2004) Myocardial ischemia, stunning, inflammation, and apoptosis during cardiac surgery: A review of evidence. Eur J Cardiothorac Surg 25(3): 304-311.
- Polderman KH, Girbes AR (2004) Severe electrolyte disorders following cardiac surgery: A prospective controlled observational study. Crit Care 8(6): R459-R466.
- He D, Sznycer-Taub N, Cheng Y, McCarter R, Jonas RA, et al. (2015) Magnesium lowers the incidence of postoperative junctional ectopic tachycardia in congenital heart surgical patients: Is there a relationship to surgical procedure complexity? Pediatr Cardiol 36(6): 1179-1185.
- Delaney JW, Moltedo JM, Dziura JD, Kopf GS, Snyder CS (2006) Early postoperative arrhythmias after pediatric cardiac surgery. J Thorac Cardiovasc Surg 131(6): 1296-1300.
- Grosse-Wortmann L, Kreitz S, Grabitz RG, Vazquez-Jimenez JF, Messmer BJ, et al. (2010) Prevalence of and risk factors for perioperative arrhythmias in neonates and children after cardiopulmonary bypass: continuous holter monitoring before and for three days after surgery. J Cardiothorac Surg 5: 85.
- Nelsona JS, Vanja S, Maul TM, Whitham JK, Ferns SJ (2019) Early arrhythmia burden in pediatric cardiac surgery fast-track candidates: Analysis of incidence and risk factors. progress in pediatric cardiology 52: 8-12.
- Kabbani MS, Al Taweel H, Kabbani N, Al Ghamdi S (2017) Critical arrythmia in postoperative cardiac children recognition and management. Avicenna J Med 7(3): 88-95.
- Walsh EP, Cecchin F (2007) Arrythmias in Adult Patients with Congenital Heart disease. Circulation 115(4): 534-545.
- Khairy P, Balaji S (2009) Cardiac Arrhythmias in Congenital Heart Diseases. Indian Pacing Electrophysiol J 9(6): 299-317.
- Niwa K (2018) Adult Congenital Heart Disease with Pregnancy. Korean Circ J 48(4): 251-276.
- Tateno S, Niwa K, Nakazawa M, Akagi T, Shinohara T, et al. (2003) Arrhythmia and conduction disturbances in patients with congenital heart disease during pregnancy: multicenter study. Circ J 67(12): 992-997.
- Khairy P (2008) EP challenges in adult congenital heart disease. Heart Rhythm 5(10): 1464-1472.
- Attenhofer Jost CH, Connolly HM, Edwards WD, Hayes D, Warnes CA, et al. (2005) Ebstein’s anomaly – review of a multifaceted congenital cardiac condition. Swiss Med Wkly 135(19-20): 269-281.
- Moore JP, Cho D, Lin JP, Lluri G, Reardon LC, et al. (2018) Implantation techniques and outcomes after cardiac resynchronization therapy for congenitally corrected transposition of the great arteries. Heart Rhythm 15(12): 1808-1815.
- Cherukupalli R, Miro S, Movva S, Patil P, Gajiwala N, et al. (2014) Double Intervention in Single Sitting: Percutaneous Device Closure and Permanent Pacemaker Implantation in a Patient with Atrial Septal Defect. IJCM 5(20): 1311-1315.
- Ming-ChunYang, Jiunn-Ren Wu (2018) Recent review of transcatheter closure of atrial septal defect. The Kaohsiung Journal of Medical Sciences 34(7): 363-369.
- Nehgme RA, Huddleston AR, Cheatham JP (2009) Cheatham Progression to late complete atrioventricular block following amplatzer device closure of atrial septal defect in a child. Pediatr Cardiol 30(3): 367-370.
- Spence MS, Qureshi SA (2005) Complications of transcatheter closure of atrial septal defects. Heart 91(12): 1512-1514.
- Hoyer AW, Balaji S (2007) The safety and efficacy of ibutilide in children and in patients with congenital heart disease. Pacing Clin Electrophysiol 30(8): 1003-1008.
- Wells R, Khairy P, Harris L, Anderson CC, Balaji S (2009) Dofetilide for Atrial Arrhythmias in Congenital Heart Disease: A Multicenter Study. Pacing Clin Electrophysiol 32(10): 1313-1318.
- Shah MJ, Jones TK, Cecchin F (2004) Improved localization of right-sided accessory pathways with microcatheter-assisted right coronary artery mapping in children. J Cardiovasc Electrophysiol 15(11): 1238-1243.
- Reich JD, Auld D, Hulse E, Sullivan K, Campbell R (1998) The Pediatric Radiofrequency Ablation Registry's experience with Ebstein's anomaly. Pediatric Electrophysiology Society. J Cardiovasc Electrophysiol 9(12): 1370-1377.
- Hebe J (2000) Ebstein's anomaly in adults. Arrhythmias: diagnosis and therapeutic approach. Thorac Cardiovasc Surg 48(4): 214-219.
- Khairy P, Stevenson WG (2009) Catheter ablation in tetralogy of Fallot. Heart Rhythm 6(7): 1069-1074.






























