Plasma Levels of SDF-1 are Acutely Correlated with Adrenergic Agonists and Modulated by β-Blockers Interactions in Hypertensive Individuals
Bruna Eibel1, Melissa Kristocheck1, Bruna Nórdio1,2, Karina Rabello Casali3, Eduardo Costa Duarte Barbosa1, David Brasil4, Maria Cláudia Irigoyen1,5, Renato Abdala Karam Kalil1,2 and Melissa Medeiros Markoski2
1Laboratório de Investigação Clínica (LIC), Fundação Universitária de Cardiologia (IC/FUC), Brazil
2Universidade Federal de Ciências da Saúde de Porto Alegre (UFCSPA), Brazil
3Universidade Federal de São Paulo (UNIFESP), Brazil
4Faculdade de Ciências Médicas de Minas Gerais (FCMMG)/Universidade Federal de Lavras (UFLA), Brazil
5Universidade de São Paulo (USP), Brazil
Submission: August 09, 2019; Published: August 28, 2019
*Corresponding author: Melissa M Markoski, Universidade Federal de Ciências da Saúde de Porto Alegre Sarmento Leite, 245 - Porto Alegre, RS, Brazil
How to cite this article: Bruna Eibel, Melissa K, Bruna N, Karina R C, Eduardo C D B et all.Plasma Levels of SDF-1 are Acutely Correlated with Adrenergic Agonists and Modulated by β-Blockers Interactions in Hypertensive Individuals. J Cardiol & Cardiovasc Ther. 2019; 14(5): 555897. DOI: 10.19080/JOCCT.2019.14.555897
Abstract
Background: To examine the acute interference of β-blockers with plasma chemokine stromal cell-derived factor-1 (SDF-1), epinephrine and norepinephrine levels, as well as cardiovascular autonomic parameters.
Methods: We investigated a cross-sectional sample comprising individuals with hypertension (HTN, n=18) and ischemic heart disease (IHD, n=18) under regimens of either atenolol, carvedilol, metoprolol or propranolol, and healthy adults (CG, n=16). We measured plasma levels of SDF-1 and recorded R-R intervals (heart rate [HR] variability) and systolic blood pressure [SBP] in the IHD and HTN groups prior to drug administration and 3 hours post-administration; the CG group was assessed at a single time point.
Results: Following drug administration SBP (mean ± SD) decreased in both groups, ICD (from 138.80 to 131.65mmHg) and HTN (147.06 to 141.19mmHg), p<0.05, and HR variability increased in the IHD (from 846.12 to 1426.56ms2) and in the HTN (1,085.89 to 1,919.93ms2), p<0.05. Baseline SDF-1 levels were significantly different in the IHD and the HTN (2,410.6 and 4,904.4pg/mL, respectively, p=0.0011). Post drug administration SDF-1 levels were lower in ICD participants under atenolol regimen compared to those on other β-blockers (1,496.2; 4,239.4pg/mL, p=0.039).
Conclusion: β-blockers may interfere with the systemic availability of SDF-1 and thus may interfere with stem cell homing and the relationship of SDF-1/catecholamines, promoting hemodynamic and autonomic responses.
Keywords: Systemic hypertension; Coronary artery disease; β-blockers; Stem cell homing; SDF-1
Abbreviations: IHD: Ischemic Heart Disease; HR: Heart Rate; SBP: Systolic Blood Pressure; HT: Hypertension; CAD: Coronary Artery Disease; ACE: Angiotensin Converting-Enzyme; ARB: Angiotensin Receptor Blockers; SBP: Systolic Blood Pressure; BP: Blood Pressure
Introduction
Hypertension (HT) is the most prevalent asymptomatic condition affecting nearly 1 billion people worldwide [1,2]. It is one of the main risk factors of Ischemic Heart Disease (IHD) [3] and also commonly diagnosed in primary care; yet, full treatment effectiveness still needs improvement [4]. Drug treatments for Coronary Artery Disease (CAD) and HTN comprise several classes of drugs such as β-blockers, statins, calcium channel blocker, Angiotensin Converting-Enzyme (ACE) inhibitors, Angiotensin Receptor Blockers (ARB), antiplatelet agents and antianginal agents [5-7].
Pharmacologic agents used to treat hypertension and CAD may act in cell signaling pathways involved in major cellular responses such as cell proliferation and differentiation as well as in cell homing [8]. Cell homing is a mechanism that promotes the detection of damaged cells and tissues by undifferentiated stem cells and then cell migration, proliferation and differentiation and replacement of apoptotic or necrotic dead cells [7]. This process is activated the chemokine stromal cell-derived factor-1 (SDF-1) that is released and binds to its specific receptors CXCR-4 and CXCR-7 located on the surface of stem cells, lymphocytes and neurons [9]. CXCR-4, as well as β-adrenergic receptors, binds to G-protein subunits and their interaction leads to the activation of signaling pathways resulting in chemotaxis [7] and other cellular processes.
β-blockers are known to be beneficial in treating HTN. They act by antagonizing the actions of β-adrenergic receptors, which are functionally coupled to G-proteins and activated by the catecholamines (epinephrine and norepinephrine) adrenaline and noradrenaline [5,10,11] that are produced in the adrenal medulla to exert cardiovascular autonomic control. Experimental observations have shown that these agents have the capacity of interfering with cell homing by reducing SDF-1 levels and the expression of its receptor CXCR-4 [8]. Individuals with ischemic heart disease and HTN usually take multiple drugs and this may significantly impact therapies involving cardiac repair and regeneration [12]. Therefore, the present study aimed to examine the interference of β-blockers on SDF-1 serum marker as well as their correlation with catecholamine levels, autonomic control, and Systolic Blood Pressure (SBP) in the IHD, HTN and control groups.
Methods
Study design and sample
We conducted a cross-sectional study approved by the Research Ethics Review Committee (ERC) of Instituto de Cardiologia do Rio Grande do Sul/Fundação Universitária de Cardiologia (IC/FUC), Porto Alegre, Brazil. All participants signed a free informed consent form in compliance with resolution 466/12.
The study sample was divided into three groups:
(i) a hypertensive group (HTN; n = 18);
(ii) an Ischemic Heart Disease group (IHD) with systolic dysfunction (ejection fraction <55%) (n = 18);
(iii) a control group (CG, n = 16) of healthy adults with no cardiovascular drug prescription.
The inclusion criteria comprised IHD or HTN patients aged 45 to 75 years on a therapeutic regimen with a β-blocker (either atenolol, carvedilol, metoprolol succinate/tartrate or propranolol) for at least 30 days. Venous blood samples were collected and stored in EDTA tubes prior to drug administration and 3 hours post-administration (e.g., time to peak plasma concentration [Tmax] for atenolol equals 2.2±0.27 hours). Concurrently, we also recorded R-R intervals, Heart Rate (HR) variability, and SBP. β-blockers and other drugs, with cardiovascular effects, were administered as prescribed for each participant at IC/FUC Clinical Research Laboratory under the supervision of the principal investigator.
Enzyme-linked Immunosorbent Assay (ELISA)
Venous blood samples were centrifuged at 2000 rpm for 10 minutes at 4°C for plasma separation and stored at –20°C until use. Plasma levels of SDF-1 (isoforms α) and catecholamines (adrenaline and noradrenaline) were measured by Enzyme-Linked Immunosorbent Assay (ELISA) using commercial kits (Quantikine, R&D Systems; DIAsource Immunoassays) in accordance with the manufacturer's instructions. Optical densities were measured in a spectrophotometer (Spectramax M2e, Molecular Devices) and quantified using a 4-parameter linear regression model (Excel, Microsoft). Data were expressed in picogram of protein per milliliter (pg/mL).
Assessment of cardiovascular autonomic control
All participants were initially informed about the study protocol and asked to rest for 15 minutes. The recordings were made in supine position. Arterial Blood Pressure (BP) and Heart Rate (HR) were continuously monitored using a sensor placed on the middle finger connected to a non-invasive BP monitor (Ohmeda 2300, Monitoring Systems, Englewood, CO, USA). Measurements were obtained using a PowerLab® device (ADInstruments Pty Ltd, Australia). Autonomic control of cardiac chronotropic function was measured using power spectral analysis (PSA) of heart rate variations. Calculations were made with an autoregressive model applied to stationary time series of 300 beats [13]. The power spectrum was split into low-frequency (LF, 0.03-0.15 Hz) and high-frequency (HF, 0.15-0.40 Hz) bands in normalized units to minimize the effects of the Very Low-Frequency (VLF) band, and LF/HF ratio to reflect a sympatho vagal balance [14].
Statistical analysis
Preliminary analyses were conducted using the Statistical Package for Social Sciences (SPSS, version 23.0) and further analyses were performed using statistical software BioEstat (version 5.0). Parametric data were described as means and standard deviations, and non-parametric continuous data were described as medians and interquartile ranges. The Student’s t-test was performed for comparison of means of two groups and ANOVA test for inter-group comparisons of the variables, followed by Tukey’s multiple comparison analysis. For quantitative comparison of protein expression between the study groups at each time point, we conducted the Kruskal-Wallis test followed by the Student-Newman Keuls multiple comparison test. We assessed protein expression at different time points in each group with the non-parametric Wilcoxon-Mann-Whitney test and the association between qualitative variables in each group using the chi-square test. We assessed the non-parametric measure of correlation between protein expression and the drugs administered using Spearman's correlation coefficient. The statistical significance level was set at p<0.05.
Results
Table 1 shows demographic information of participants. The participants’ age (p = 0.02) and the prevalence of sedentary lifestyle (p = 0.023) were higher in the IHD and HTN groups compared to CG. Diabetes mellitus was more prevalent among the HG compared to the other two groups (p = 0.044). As for vascular disease the baseline characteristics differ in previous underlying conditions (p ≤ 0.001). Table 1 also details all drugs taken by participants, including β-blockers (p ≤ 0.001).
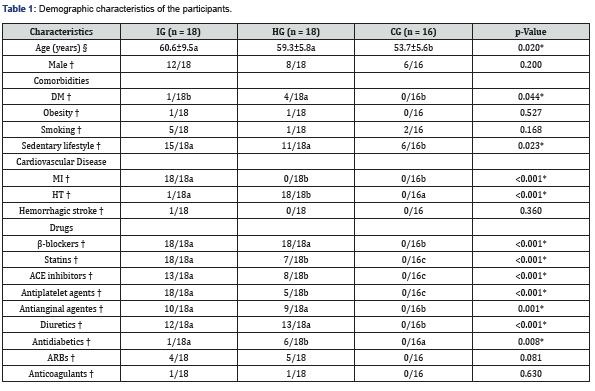
Drug administration reduced noradrenaline levels in individuals with IHD
Figure 1 shows the plasma catecholamine levels in all three groups at baseline, prior to drug administration and 3 hours post-administration. The levels of adrenaline were lower in those taking β-blockers compared to the CG, but this difference was not significant (p = 0.143, Figure 1A). The levels of noradrenaline varied over time in the IG compared to the other groups (Figure 1B). Forthe IG, the level of noradrenaline dropped following drug administration, from 429.8 to 389.5pg/mL (p = 0.006). This same group showed higher catecholamine levels prior to drug administration compared to the other groups (429.8pg/mL [IG];395.8pg/mL [HG], p = 0.0034; and 429.8pg/mL [IG] and 367.7pg/mL [CG], p <0.0001). After drug administration, noradrenaline levels did not differ among the groups.
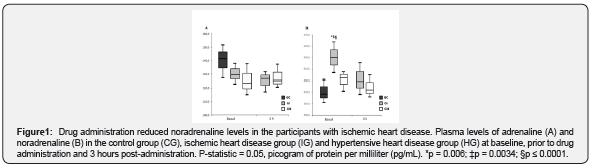
Individuals with IHD showed lower SDF-1 levels compared to those with HT prior to drug administration
Figure 2 shows the plasma SDF-1 levels in the CG and prior to drug administration and 3 hours post-administration in the IG and the HG. The time elapsed post-administration was not able to elicit differences in the chemokine levels (p = 0.266), although there was a slight increase in the IG. The IG also shower lower SDF-1 levels at baseline compared to the HG (2,410.6pg/mL [IG] and 4,904.4pg/mL [HG], p = 0.0011), but this difference was no longer seen following drug administration.
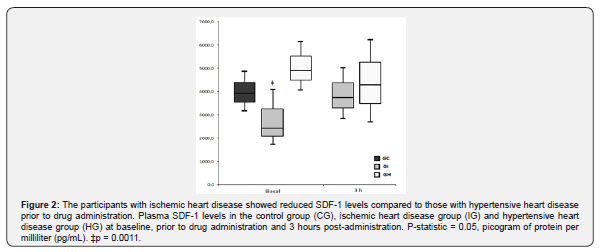
SDF-1 and catecholamine levels are correlated in individuals with HT and drugs modulate these levels
We conducted a correlation analysis to assess the relationship between SDF-1 and catecholamine levels prior to and post drug administration in the IG and the HG (Figure 3). There was found no relationship in the IG. However, a correlation of SDF-1 with noradrenaline levels at baseline was seen in the HG (r = 0.512, p = 0.030, Figure 3A) and with both adrenaline and noradrenaline 3 hours post-administration showing a direct correlation with adrenaline (r = 0.504; p = 0.033, Figure 3B) and an inverse correlation with noradrenaline (r = –0.449, p = 0.050, Figure 3C).
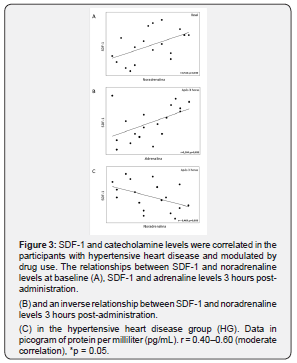
Atenolol and metoprolol interfere with SDF-1 levels
Figure 4 presents the results of SDF-1 modulation prior to and post administration of atenolol and metoprolol. These two drugs were assessed separately from the other β-blockers in the IG and the HG. Three hours post-administration SDF-1 levels were lower in the IG taking atenolol compared to those taking other β-blockers (1,496.2pg/mL; 4,239.4pg/mL, p = 0.039, Figure 4A). In contrast, when we assessed the HG participants taking metoprolol compared those taking other β-blockers, they showed higher SDF-1 levels prior to drug administration (9,891.8 – 4,738.1pg/mL, p = 0.050, Figure 4B) with a decrease after drug use.
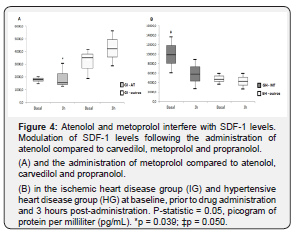
β-blockers promote hemodynamic and autonomic responses in individuals with IHD and HT 3 hours post-administration
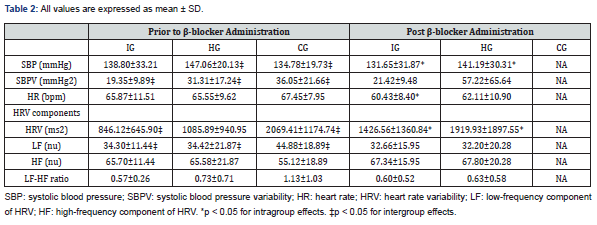
Table 2 shows hemodynamic and autonomic parameters in the participants prior to and post drug administration. Intra-group analyses that were statistically significant showed a reduction in SBP in the IG (from 138.80 to 131.65mmHg, p <0.05) and the HG (from 147.06 to 141.19mmHg, p <0.05) and an increase in the HR variability in the IG (846.12 to 1,426.56ms2; p <0.05) and the HG (1,085.89 to 1,919.93ms2, p <0.05). Prior to drug administration, compared to controls, there were significant differences in SBP (134.78mmHg [CG], 147.06mmHg [HG], p <0.05), systolic blood pressure variability (SBPV) (36.05mmHg2 [CG], 19.35mmHg2 [IG], p <0.05), Heart Rate Variability (HRV) (2,069.41ms2 [CG], 846.12ms2 [IG], 1,085.89ms2 [HG], p <0.05) and LF (44.88 [CG], 34.30 [IG],34.42 [HG]; all unitless, p <0.05).
Discussion
The purpose of our study was to examine the interference of β-blockers and other drugs used in combination with plasma SDF-1 and catecholamine levels and cardiovascular autonomic control in individuals with HT comparing with IHD and control groups. Signaling pathways of β-blockers are known to share common cell homing molecules such as G-proteins, G-protein coupled receptor kinases and β-arrestins [15-17]. This study was designed to assess acute effects of cardiovascular drugs especially β-blockers on signaling of cells involved in homing and plasma catecholamines as well as sympathetic and parasympathetic cardiac modulation. Our protocol was designed considering that peak levels of β-blockers are reached at 2-3 hours [18] (instead of peak levels of SDF-1that are reached within 24 hours to 7 days) [17].
We chose to examine individuals with HT because the use of β-blockers is part of treatment strategies for this condition. Purposing the comparison with ischemic and healthy controls, our goal was identified different responses about the SDF-1 behavior in these clinical conditions. Corroborating the literature, both groups showed a higher prevalence of sedentary lifestyle compared to controls. Sedentary lifestyle is an established risk factor for heart conditions [3]. The use of an ischemic and a control groups allowed to determine high and baseline values of the molecules analyzed in a population with severe or no heart disease.
Previous findings have pointed to a direct effect of β-blockers on circulating catecholamines especially adrenaline [19]. The combined assessment of adrenaline with SDF-1 levels showed a correlation with this major cell homing factor 3 hours post-administration in the HG. It has been previously described that signaling pathways of catecholamines can promote CD34+ cell adhesion and migration by acting directly on hematopoietic stem and progenitor cells, probably through the canonical Want signaling pathway [20].
The IG showed a significant reduction in noradrenaline levels from baseline to 3 hours post-administration. This finding demonstrates that the intervention (drug administration) induced the sensitization of noradrenaline receptors to systemically released noradrenaline, which has an average life of 2 to 2.5 minutes (but it may vary individually) [21]. Moreover, β-blockers have been reported to promote baroreceptor readaptation and catecholamine reduction in nerve synapses [22], thus supporting our results. We also found that noradrenaline levels in the IG at baseline were different from those in the other groups. It suggests that individuals with IHD, even when they are on antihypertensive drugs, have increased levels of this potent vasopressor [23], which may be explained by an intensified action of vasoconstrictors in heart ischemia [5]. The relationship of noradrenaline with SDF-1 was seen in the HG at baseline and 3 hours post-administration: plasma SDF-1 levels increased as noradrenaline decreased. It suggests an influence of the circadian cycle and autonomic nervous system on SDF-1, which is corroborated by Spiegel et al. [20] finding that the levels of this chemokine are deeply influenced by nocturnal decline-wake states [20].
The assessment of hemodynamic and autonomic parameters revealed a clinically significant reduction in SBP in the treated groups. It demonstrates the acute effects of cardiovascular drugs, which were associated with increased the HR variability in these groups - a phenomenon that occurs to maintain BP stable [13]. Compared to the other two groups prior to drug administration, the CG showed well-controlled BP, greater HR variability and greater intensification of the LF component that reflects cardiac sympathetic activation indicating normal physiological responses in healthy individuals [14].
Stem cell homing is a physiological process that occurs in almost all tissues and in certain pathological states, either acute or chronic [24], for cell replacement, with the release of high amounts of SDF-1. In our study, the IG showed increased levels of SDF-1 prior to and post drug administration. Given that cardiac function is impaired due to ischemia in this group, our results show that β-blockers desensitized cell homing receptors, possibly impairing SDF-1 binding to its CXCR-4 receptor and leading to high plasma levels of this protein. This finding supports those reported by Chang et al. [25] suggesting that high and low levels of SDF-1 following primary coronary angioplasty are predictive of mobilization of endothelial progenitor cells [25]. A comparison of the IG and HG revealed different baseline SDF-1 levels, which may be explained by pathophysiological characteristics specific to each condition.
Chemokines produce a chemoattractant gradient for other immune cells (macrophages and leukocytes) and progenitor cells (endothelial and mesenchymal cells) that can promote tissue regeneration. Factors such as hypoxia, endothelial dysfunction and oxidative stress trigger immune and progenitor cell homing to establish the reparative processes [26]. Additionally, it has been suggested that SDF-1 not only acts as a chemotactic factor, but also acts on the retention of proangiogenic cells in the perivascular region [27]. However, in the absence of this initial step during cell homing, therapies with the use of growth factor would not be effective because cells would not function in a paracrine manner and thus act in regenerative processes. These data are corroborated by a recent review study conducted by our group showing that β-blockers adversely interfere with the SDF-1/CXCR-4/CXCR-7 axis [27] with consequent reduced rates of stem and progenitor cell proliferation and adhesion (cell homing).
We obtained important results to support the interference of β-blockers when we assessed the drugs separately. The participants taking atenolol had lower SDF-1 levels 3 hours post-administration. The finding that atenolol interfere in the main axis of cell homing is also supported by Sharifpanah et al. [28] study that showed β-blockers can interfere with stem cell differentiation into endothelial cells. In conclusion, our examination of the interference of cardiovascular drugs especially β-blockers in proliferative and neurotransmitter pathways showed that drug therapy can affect plasma levels of chemokines involved in cell homing and catecholamines from the sympathetic nervous system by desensitizing specific receptors and interfering with cell signaling pathways and thus affecting the endpoints of these pathways, i.e., cellular motility and vascular function control (vasoconstriction/vasodilation). The study findings can significantly contribute to the knowledge of interaction effects between conventional and modern therapies when they are administered in combination without a specific handling to ensure optimal outcomes.
Informed Consent and Ethics
Informed consent: Informed consent was obtained from all individual participants included in the study.
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Acknowledgement
The authors would like to thank all participants of this study.
Funding
This study was funded by National Council of Scientific and Technological Development (CNPq) [Grant number: MCT/CNPq process number 014/2011]. Bruna Eibel received funding for doctoral students from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul (FAPERGS).
Data Availability
The data used to support the findings of this study are included within the article.
References
- Trudel X, Brisson C, Milot A, Masse B, Vézina M (2016) Adverse psychosocial work factors, blood pressure and hypertension incidence: repeated exposure in a 5-year prospective cohort study. J Epidemiol Community Health 70(4): 402-408.
- Chockalingam A, Campbell NR, Fodor JG (2006) Worldwide epidemic of hypertension. Can J Cardiol 22(7): 553-555.
- Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, et al. (2003) The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 289(19): 2560-2572.
- Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, et al. (2010) Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association's strategic Impact Goal through 2020 and beyond. Circulation 121(4): 586-613.
- DiNicolantonio JJ, Fares H, Niazi AK, Chatterjee S, D'Ascenzo F, et al. (2015) β-Blockers in hypertension, diabetes, heart failure and acute myocardial infarction: a review of the literature. Open Heart 2(1): e000230.
- DiNicolantonio JJ, Lavie CJ, Fares H, Menezes AR, O'Keefe JH (2013) Meta-analysis of carvedilol versus beta 1 selective beta-blockers (atenolol, bisoprolol, metoprolol, and nebivolol). Am J Cardiol 111(5): 765-769.
- Schaun MI, Eibel B, Kristocheck M (2016) Cell Therapy in Ischemic Heart Disease: Interventions That Modulate Cardiac Regeneration. Stem Cells Int 2016: 2171035.
- Zou HX, Jia J, Zhang WF, Sun ZJ, Zhao YF (2013) Propranolol inhibits endothelial progenitor cell homing: a possible treatment mechanism of infantile hemangioma. Cardiovasc Pathol 22(3): 203-210.
- Imitola J, Raddassi K, Park KI, Mueller FJ, Nieto M, et al. (2004) Directed migration of neural stem cells to sites of CNS injury by the stromal cell-derived factor 1alpha/CXC chemokine receptor 4 pathway. Proc Natl Acad Sci U S A 101(52): 18117-18122.
- Prins KW, Neill JM, Tyler JO, Eckman PM, Duval S (2015) Effects of Beta-Blocker Withdrawal in Acute Decompensated Heart Failure: A Systematic Review and Meta-Analysis. JACC Heart Fail 3(8): 647-653.
- DiNicolantonio JJ, Hackam DG (2012) Carvedilol: a third-generation β-blocker should be a first-choice β-blocker. Expert Rev Cardiovasc Ther 10(1): 13-25.
- Ganju RK, Brubaker SA, Meyer J, Dutt P, Yang Y, et al. (1998) The alpha-chemokine, stromal cell-derived factor-1alpha, binds to the transmembrane G-protein-coupled CXCR-4 receptor and activates multiple signal transduction pathways. J Biol Chem 273(36): 23169-23175.
- Chua KC, Chandran V, Acharya UR, Lim CM (2008) Cardiac state diagnosis using higher order spectra of heart rate variability. J Med Eng Technol 32(2): 145-155.
- Montano N, Porta A, Cogliati C, Costantino G, Tobaldini E, et al. (2009) Heart rate variability explored in the frequency domain: a tool to investigate the link between heart and behavior. Neurosci Biobehav Rev 33(2): 71-80.
- McCormick PJ, Segarra M, Gasperini P, Gulino AV, Tosato G (2009) Impaired recruitment of Grk6 and beta-Arrest in 2 causes delayed internalization and desensitization of a WHIM syndrome-associated CXCR4 mutant receptor. PLoS One 4(12): e8102.
- Wang J, Guan E, Roderiquez G, Calvert V, Alvarez R, et al. (2001) Role of tyrosine phosphorylation in ligand-independent sequestration of CXCR4 in human primary monocytes-macrophages. J Biol Chem 276(52): 49236-49243.
- Bromage DI, Davidson SM, Yellon DM (2014) Stromal derived factor 1α: a chemokine that delivers a two-pronged defence of the myocardium. Pharmacol Ther 143(3): 305-315.
- Weber MA (2005) The role of the new beta-blockers in treating cardiovascular disease. Am J Hypertens 18(12 Pt 2): 169S-176S.
- Pellinger TK, Halliwill JR (2007) Effect of propranolol on sympathetically mediated leg vasoconstriction in humans. J Physiol 583(Pt 2): 797-809.
- Spiegel A, Shivtiel S, Kalinkovich A, Ludin A, Netzer N, et al. (2007) Catecholaminergic neurotransmitters regulate migration and repopulation of immature human CD34+ cells through Wnt signaling. Nat Immunol 8(10): 1123-1131.
- Matsukawa R, Hirooka Y, Ito K, Sunagawa K (2013) Inhibition of neuregulin-1/ErbB signaling in the rostral ventrolateral medulla leads to hypertension through reduced nitric oxide synthesis. Am J Hypertens 26(1): 51-57.
- Pedersen ME, Cockcroft JR (2006) The latest generation of beta-blockers: new pharmacologic properties. Curr Hypertens Rep 8(4): 279-286.
- López-Sendón J, Swedberg K, McMurray J, Tamargo J, Maggioni AP, et al. (2004) Expert consensus document on beta-adrenergic receptor blockers. Eur Heart J 25(15): 1341-1362.
- Schächinger V, Aicher A, Döbert N, Röver R, Diener J, et al. (2008) Pilot trial on determinants of progenitor cell recruitment to the infarcted human myocardium. Circulation 118(14): 1425-1432.
- Chang LT, Yuen CM, Sun CK, Wu CJ, Sheu JJ, et al. (2009) Role of stromal cell-derived factor-1alpha, level and value of circulating interleukin-10 and endothelial progenitor cells in patients with acute myocardial infarction undergoing primary coronary angioplasty. Circ J 73(6): 1097-1104.
- Kanki S, Segers VF, Wu W, Kakkar R, Gannon J, et al. (2011) Stromal cell-derived factor-1 retention and cardioprotection for ischemic myocardium. Circ Heart Fail 4(4): 509-518.
- Askari AT, Unzek S, Popovic ZB, Goldman CK, Forudi F, et al. (2003) Effect of stromal-cell-derived factor 1 on stem-cell homing and tissue regeneration in ischaemic cardiomyopathy. Lancet 362(9385): 697-703.
- Sharifpanah F, Saliu F, Bekhite MM, Wartenberg M, Sauer H (2014) β-Adrenergic receptor antagonists inhibit vasculogenesis of embryonic stem cells by downregulation of nitric oxide generation and interference with VEGF signalling. Cell Tissue Res 358(2): 443-452.






























