Double Pseudoaneurysms of the Aortic Bulbus after Aortic Valve Replacement Surgery
Krasic Stasa1* and Zivkovic Igor2
1Department of Cardiology, Mother and Child Health Care Institute of Serbia “Dr.Vukan Cupic”, Serbia
2Cardiovascular Institute, Serbia
Submission: April 08, 2019; Published: April 24, 2019
*Corresponding author: Krasic Stasa, Department of Cardiology, Mother and Child Health Care Institute of Serbia “Dr Vukan Cupic”, R. Dakica St. 6-8, 11070 Belgrade, Serbia
How to cite this article:Krasic S, Zivkovic I. Double Pseudoaneurysms of the Aortic Bulbus after Aortic Valve Replacement Surgery. J Cardiol & Cardiovasc Ther. 2019; 13(5): 555874. DOI: 10.19080/JOCCT.2019.13.555874
Abstract
Pseudoaneurysm of the ascending aorta is a rare and very dangerous complications after cardiac procedures with heterogeneous clinical presentation.
We presented a patient with double pseudoaneurysms of the ascending aorta with origin from the right sinus which completely occluded right coronary artery and produced ischemic heart symptoms. The patient underwent to the surgery and the postoperative recovery was uneventful.
To the best of our knowledge, this is the first reported case of double pseudoaneurysms arising from the right edge of the aortic bulbus.
Keywords: Pseudoanerysms; Aorta; Angina
Introduction
EA pseudoaneurysm of the ascending aorta represents a rare, life-threatening complication of cardiac surgical interventions [1].
The symptomatology of pseudoaneurysm of ascending aorta (PAA) is clinically heterogeneous. The anatomical localization of expansion which leads to compression or erosive effect on the structures of the mediastinum induces a corresponding clinical picture, which becomes even more versatile if there are two PAA. Contrast computer tomography (CT) scanning, magnetic resonance imaging, and echocardiography are diagnostic methods of PAA, with high sensitivity and specificity. Surgical treatment is mandatory because high risk of death exist [2].
We presented a patient with double pseudoaneurysms of the ascending aorta with origin from the right sinus which completely occluded right coronary artery and produced ischemic heart symptoms.
Case Report
A 61-year old female was admitted with eight months history of fatigue, a sense of heart palpitation, irritating dry cough and hoarse voice. Тhe patient was operate four years ago, when stenotic aortic valve was replaced with mechanical prosthesis.
Transthoracic echocardiography revealed 4-cm-wide cystic formation as well as a smaller round cavity behind it. The origin of cyst was from the right and non-coronary sinus of Valsava. Color Doppler showed communication between the aorta and the larger cavity during systole.
Transesophageal echocardiography (TEE) found a cystic formation in the front and right part in relation to the root of the aorta, in front of the right atrium and chamber, 6.8 cm in diameter in the superior-inferior direction, and 4.5 cm in the mediolateral direction, divided into two cavities, a larger front and a smaller rear. Mural thrombosis was noted in the cavities. Also, a communication between the pseudoaneurysm and the aorta was clearly noted at the spot between the right and the non-coronary sinus of Valsava.
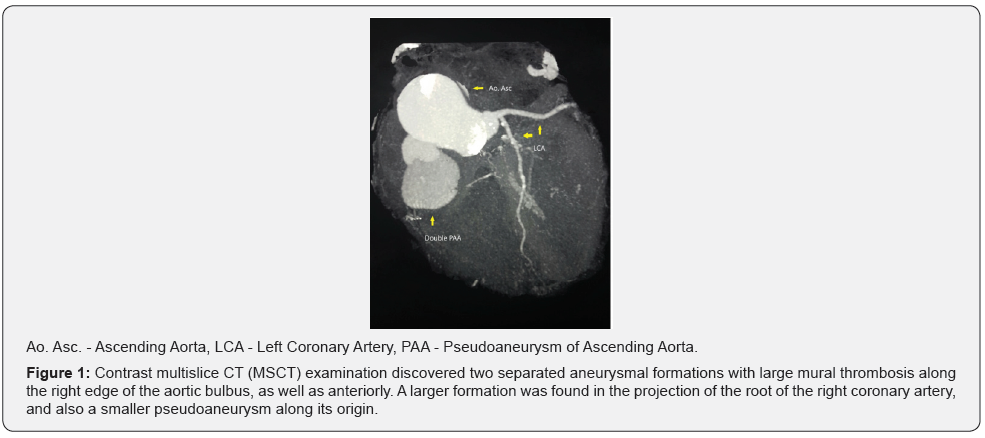
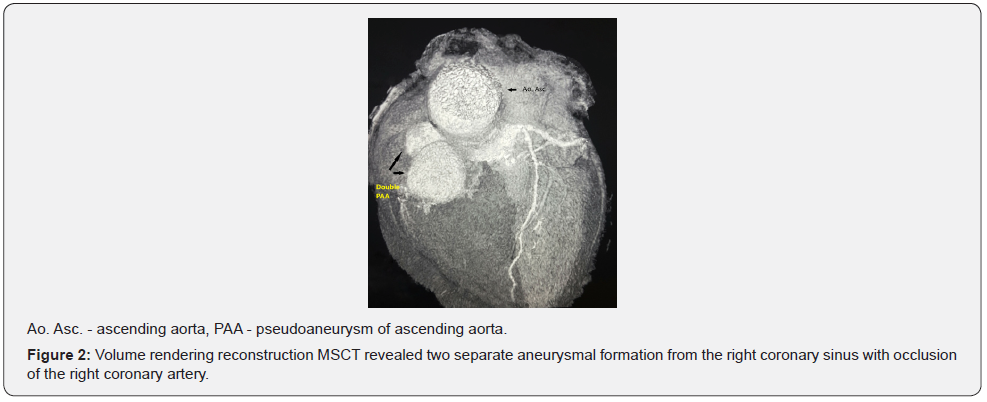
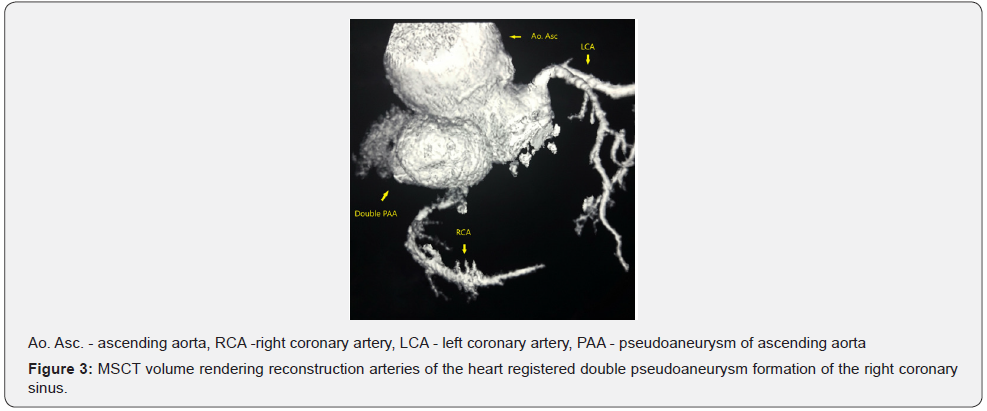
Contrast multislice CT (MSCT) examination discovered two separated aneurysmal formations with large mural thrombosis along the right edge of the aortic bulbus, as well as anteriorly (Figure 1). A larger formation was found in the projection of the root of the right coronary artery, and also a smaller pseudoaneurysm along its origin (Figure 2 & 3).
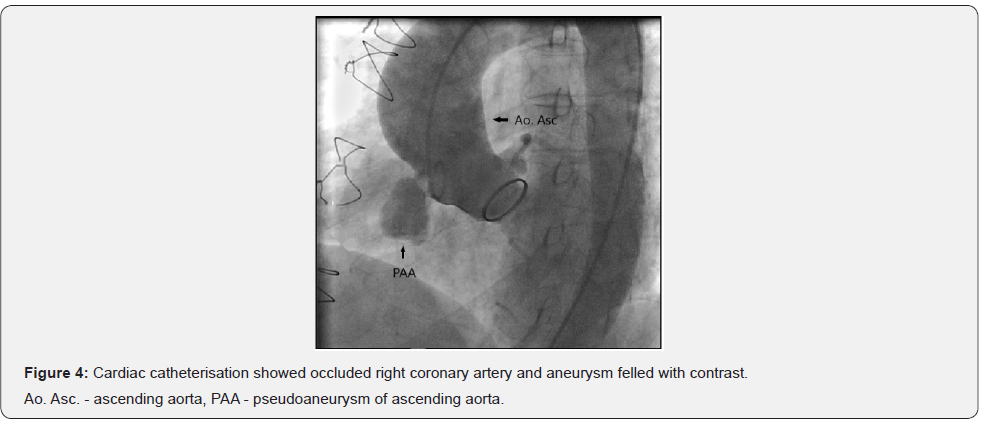
Cardiac categorization revealed two pseudoaneurysmal formations in the right part of the aortic bulbus which completely occlude the origin of the right coronary artery, which could visualised only due to retrograde flow from left coronary artery (Figure 4).
The patient was operated in deep hypothermia, without the occurrence of intraoperative complications. There were no complications during the postoperative period. The postoperative recovery was uneventful.
Discussion
The available medical literature reports an incidence of less than 0.5 %, with high mortality rate (29 - 46 %) [2,3]. Previous cardiac surgery procedure increases incidence rate more than 13% [4]. The etiology of PAA is multifactorial. The infection, connective tissue disease, chronic hypertension, aortic calcification or trauma increase the risk for creating false aneurysm. The most common surgical cause of PAA are aortic injury on the area of cannulation and cross – clamp, cardiologic needle place and on the suture lines [5]. The main pathophysiological mechanism represents the delamination of the aortic wall, and created false lumen confined only by fragile tunica adventitia [6]. Weakness of wall leads to an increased probability of spontaneous rupture which could result in mediastinal haemorrhage, acute cardiac tamponade, hypovolemic shock and death. The mechanisms of the appearance of pseudoaneurysms have not been completely clarified. Dhadval et al. [7] suggested two theories while uncovering the causes of postoperative PA [7]. According to the first theory, postoperative bleeding through the thoracic drain, which was not taken care of during the surgery, increases the risk of the appearance of aneurysms. According to the second one, sternal infection contributes to their appearance. In the case of our patient, none of the mentioned factors was present.
The most common clinical signs and symptoms of ascending aorta pseudoaneurysm are the pulsating mass, dysphagia, hoarseness, stridor, or angina due to obstruction of the coronary blood vessel or graft [6]. The occurrence of myocardial infarction caused by complete occlusion of the same is also possible. Asymptomatic PAA was described in a few patients. The most common were those who suffer from inflammatory diseases (Behcet’s disease), or those with congenital disorders (Marfan syndrome) [4]. The available literature cites the example of PAA, which induced occlusion of the right coronary artery ostium, by inducing the same clinical picture as in the shown patient [8]. The occurrence of hemoptysis and hematemesis as a result of aorto - bronchial and aorto - esophageal fistula is also described [9].
The diagnosis is set using TEE, contrast CT and nuclear magnetic resonance (NMR) imaging, cardiac categorization, and recently, contrast MSCT. Because of the possibility of visualization of perivalvular PA, NMR is considered superior to CT and TEE, but NMR is unacceptable for patients with artificial valves as well as those with a pace maker [10]. TEE and CT are used only in the evaluation of the defect on the front wall of the ascending aorta [8]. However, TEE may be falsely negative in tests on the front wall of the ascendant aorta in patients with aortic calcification, or those with artificial heart valves, due to the creation of acoustic shadows [10,11]. Ugolini et al. [11] states as the latest and most successful diagnostic method for the assessment of the anatomic details of false aneurysm the electron -beat computerized tomography (EBCT), especially in patients with artificial aortic valve and small PAA (5mm). EBCT can also be a way to see the spread of PAA, as well as their relationship with the coronary arteries and surrounding mediastinal structures [11].
Cardiac catheterization and angiography use to be the gold standard in the diagnosis of pseudoaneurysm but is no longer required due to the existence of modern non-invasive methods. It is, however, still necessary if the symptomatology is dominated by anginous ailments such as was the case with our patient in whom one of the two pseudoaneurysm completely occluded the origin of the right coronary artery [7,8]. Until about 10 years ago, due to the lack of adequate grafts in length, flexibility and curvature, the surgical treatment was the only way for the disposal of ascending aorta pseudoaneurysm. This method of treatment carried certain risks. The reopening of the chest carries the risk of fatal hemorrhage or air embolism [12]. This risk increases during the intervention of duplicate false aneurysm, as in our case. For this reason, it is necessary to carefully plan the surgical procedure and the protection of brain [13]. On the other hand, Roselli et al. [14] and Preveza et al. [15] preformed thoracic endovascular aortic procedures (TEVAR) in patients with PAA, and they concluded that endovascular technology can facilitate their treatment, especially in high-risk individuals [14,15]. Consequently, now a day endovascular procedure is limited to patients who have very high intraoperative risk [4].
Conclusion
The specificity of the case of our patient is the existence of a double pseudoaneurysm, which in itself is a rarity, but also the fact that one of the two pseudoaneurisms led to occlusion of the origin of the right coronary artery with the symptoms of unstable angina pectoris. The available methods for our country, TTE, TEE, CT and angiography as well as the selective coronary angiography were used for diagnostics, and the treatment was surgery with satisfactory outcome.
References
- Garisto DJ, Medina A, Williams BD, Carrillo RG (2010) Surgical Management of giant ascending aortic pseudoaneurysm. Tex Heart Inst J 37(6): 710-713.
- Parihar B, Choudhary LSD, Madhu AP, Alpha MK, Thankachen R, et al. (2005) Pseudoaneurysm of Ascending Aorta After Aortic Valve Replacement. Ann Thorac Surg 79(2): 705-707.
- Jung TE, Lee DH (2011) Surgery for pseudoaneurysm of the ascending aorta under moderate hypothermia. J Cardiothorac Surg 6: 125.
- Quevedo HC, Santiago-Trinidad R, Castellanos J, Atianzar K, Anwar A, et al. (2014) Systematic review of interventions to repair ascending aortic pseudoaneurysms. Ochsner J 14(4): 576-585.
- Sullivan KL, Steiner RM, Smullens SN, Griska L, Meister SG (1988) Pseudoaneurysm of the ascending aorta following cardiac surgery. Chest 93(1): 138-143.
- Dumont E, Carrier M, Cartier R, Pellerin M, Poirier N, et al. (2004) Repair of aortic false aneurysm using deep hypothermia and circulatory arrest. Ann Thorac Surg 78(1): 117-121.
- Dhadwal AK, Abrol S, Zisbrod Z, Cunningham JN Jr (2006) Pseudoaneurysms of the ascending aorta following coronary artery bypass surgery. J Card Surg 21(3): 221-224.
- Omeroglu SN, Mansuroglu D, Goksedef D, Cevat Y (2005) Ultrafast computed tomography in management of post-Bentall aortic root pseudoaneurzsm repair. Tex Heart J 32(1): 91-94.
- Razzouk A, Gundry S, Wang N, Heyner R, Sciolaro C, et al. (1993) Pseudoaneurysms of the aorta after cardiac surgery or chest trauma. Am Surg 59(12): 818-823.
- Lankipalli RS, Pellecchia M, Burke JF (2002) Magnetic resonance angiography in the evaluation of aortic pseudoaneurysm. Heart 87(2): 157.
- Ugolini P, Mousseaux E, Hernigou A, Gaux JC (2000) Infectious pseudoaneurysms suspected at echocardiography: electron-beam CT findings. Radiology 217(1): 263-269.
- Gaudino M, Alessandrini F, Canosa C, Possati G (1999) Repair of an ascending aorta pseudoaneurysm by way of superior ministernotomy. Ann Thorac Surg 67(6): 1798-1800.
- Domoto S, Koike H, Niinami H, Kimura F (2014) Double giant pseudoaneurysms of the aortic root and arch after ascending aorta replacement. J Vasc Surg 59(4): 1119.
- Roselli EE, Idrees J, Greenberg RK, Johnston DR, Lytle BW (2015) Endovascular stent grafting for ascending aorta repair in high-risk patients. J Thorac Cardiovasc Surg 149(1): 144-151.
- Preventza O, Henry MJ, Cheong BY, Coselli JS (2014) Endovascular Repair of the Ascending Aorta: When and How to Implement the Current Technology. Ann Thorac Surg 97(5): 1555-1560.






























