Hepatitis C Viral Load as a Predictor of Coronary Artery Disease Severity
Adham Abdeltawab*, Abdelsalam Gebril and Ahmed Onsi
Department of Cardiology, Faculty of Medicine, Ain Shams University, Cairo, Egypt
Submission: February 21, 2019; Published: March 14, 2019
*Corresponding author: Adham Abdeltawab, Cardiology Department, Ain Shams University Hospitals, Faculty of Medicine, Ain Shams University. Abbassia, Cairo, Egypt
How to cite this article:Adham A, Abdelsalam G, Ahmed O. Hepatitis C Viral Load as a Predictor of Coronary Artery Disease Severity.J Cardiol & Cardiovasc Ther. 2019; 13(3): 555861. DOI: 10.19080/JOCCT.2019.13.555861
Abstract
Introduction: Chronic liver disease (CLD) has high incidence and prevalence worldwide. Egypt has the highest prevalence rate of hepatitis C virus in the world. Chronic HCV could potentially lead to adverse cardiovascular outcomes.
Aim of the work: To assess the correlation between hepatitis C virus infection severity and extent of coronary artery disease among diabetic patients presenting to Ain Shams University Cath-lab for coronary angiography or PCI.
Methods: HCV severity was assessed by PCR and CAD severity was assessed using Syntax II score.
Results: PCR level was highly correlated to the absolute SS and to the SS group, yet it was not found to be significantly correlated to the presence of significant coronary lesion nor to the number of vessels affected. SS was highly correlated to the presence of significant coronary lesion and to the number of vessels affected.
Keywords: Hepatitis C; Ischemic heart disease; Syntax; Coronary; PCR
Abbrevations: CLD: Chronic Liver Disease; HCV: Hepatitis C Virus; PCR: Polymerase Chain Reaction; CAD: Coronary Artery Disease; CVE: Cardiovascular Events; PCI: Percutaneous Coronary Intervention; SS: Syntax Score II
Introduction
Chronic Liver Disease (CLD) has high incidence and prevalence worldwide [1]. The association between liver disease and the cardiovascular system has been described for more than 50 years [2,3]. Cirrhotic cardiomyopathy has been defined as a chronic cardiac dysfunction in patients with liver cirrhosis and/or portal hypertension, characterized by a sudden decrease in the cardiac contractile response to physical, pathological or pharmacological stress, but with normal cardiac output at rest [2,3]. Hepatitis C virus (HCV) chronic infection may be associated with advanced or early stage atherosclerosis. A possible association between HCV infection and carotid atherosclerosis was demonstrated [4]. Recent results indicate that seropositivity for HCV shows a positive association with carotid artery plaque and carotid intima–media thickening, independent from other risk factors for atherosclerosis [5]. Egypt has the highest prevalence rate of hepatitis C virus in the world, making it the most challenging public health problem facing the country [6]. In the Middle East almost all anti-HCV positive individuals are infected with type 4 [7]. Infectious etiologies have been hypothesized to contribute to the inflammatory cascade leading to atherosclerosis [8]. Some studies have found cross-sectional associations between HCV and cardiomyopathies, [9] coronary atherosclerosis, carotid artery plaque, [10] and increased pulse wave velocity, although not all studies support these findings.
Previous studies have shown that CAD severity and complexity assessed by SYNTAX score (SYNTAX = Synergy Between PCI With TAXUS and Cardiac Surgery) (SS), are associated to increased cardiovascular events (CVE) a factor of mortality and morbidity [11].
Efforts have been made for the prediction of CAD severity and complexity using non-invasive methods in order to identify the patients at high risk for CVE and treatment challenges before CCA [12]. Based on these prior findings, and as HCV genotype in Egypt is different than those of other parts of the world, we conducted this study to explore the relationship between HCV seropositivity and PCR and clinical outcomes among individuals with established CAD.
Objectives
The aim of this study was to assess the correlation between hepatitis C virus infection severity and extent of coronary artery disease among diabetic patients presenting to Ain Shams University Cath lab for coronary angiography or PCI.
Subjects and Methods
Research Design
This prospective cohort study was designed to determine how Hepatitis C and its severity assessed by PCR influence disease progression and severity in persons with coronary disease being assessed by Syntax Score.
Research setting
The current diagnostic accuracy study was carried out in the Cardiovascular Diseases Unit at Ain Shams University hospitals, starting November 2015 until Septemper2017.
The severity of CAD as assessed by Syntax score and the level of HCV RNA as detected by quantitative PCR were compared among sixty-one patients with positive HCV antibody test attended at Ain Shams University hospitals for elective coronary angiography.
Ethical statement
Informed consent was obtained from everyone participating in the study.
Patients: The study involved sixty-one patients presented to Ain Shams University catheterization lab for elective coronary angiography or PCI.
Inclusion criteria of the study:
Stable clinical condition.
Patient diagnosed to have HCV infection by HCV antibodies.
Indication for elective coronary angiography as per guidelines.
Exclusion criteria:
1. Patients presented with critical illness or hemodynamic instability, surgery or trauma within the previous month, known cancer, or hepatic failure.
2. Severe liver damage and cirrhosis, acute or chronic inflammatory disease, immunological disease, and history or presence of neoplastic disease.
3. Patients refusal to do catheterization and refusal to be added to the study.
4. Chronic renal failure on dialysis.
5. Patient’s ageless than18 years, or more than 80 years.
Methodology
All patients were subjected to:
I) Proper history taking:
All subjects gave a complete history which included:
Age.
Sex.
Special habits including: Smoking/tobacco (Cigarettes smoking; number of cigarettes / day and duration, other types of smoking……) and alcohol consumption.
Family history of ischemic heart disease.
Assessment of presenting complaints as chest pain (onset, course, duration, site, radiation, character, precipitating and relieving factors and associated symptoms).
Other symptoms as dyspnea on exertion
History of medical diseases: including arterial hypertension (duration, medication, controlled, not controlled)
History of drug therapy
II) Complete clinical examinations were done including:
General examination
Blood pressure measurement using mercurial sphygmomanometer, pulse rate and body temperature.
Peripheral pulse examination.
Weight, height and BMI.
Abdominal examination.
Local cardiac examination.
III) Labs:
Blood samples were collected using standard technique into tubes containing EDTA for necessary investigations.
1) Complete blood count, HCV RNA and serum creatinine levels were obtained from all patients.
2) Confirmation of positivity for hepatitis C; HCV serostatus were determined from documentation of a prior positive HCV antibody test in the patient’s medical record.
3) Measurement of PCR: estimation of serum HCV RNA by quantitative viral load tests (Polymerase chain reaction (PCR).
Quantitative viral load tests (Polymerase Chain Reaction (PCR) levels were measured with the American; The StepOne™ Real- Time PCR System API7000, applied biosystems, Life Technologies Corporation, USA.
Where:
• Under detectable level, < 50 IU was considered negative,
• Low viremia < 100,000 IU/ml,
• Moderate viremia from 100,000 to 1,000,000 IU/ml and,
• Marked viremia >1,000,000IU/ml [13].
IV) Coronary angiography
All patients underwent elective coronary angiography in different views and projections using Seldinger technique. Significant lesion was defined as a 50% or greater stenosis in the luminal diameter of any major epicardial coronary artery. The presence of significant lesions was determined based on visual estimation.
Syntax score was calculated for every patient with significant CAD.
Syntax score is mainly associated with CAD complexity and it was calculated using dedicated software, which integrates two components (a) morphological features of each lesion such as dominance, chronic total occlusion (CTO), bifurcation, trifurcation, tortuosity, heavy calcification, lesion length, presence of thrombus, aorto-ostial and diffuse lesions, and (b) weighting factors of lesions based on myocardial area distal to lesion. Lesions with ≥ 50% luminal obstruction in vessels with a diameter ≥1.5 mm were added to provide SS [14].
All angiograms were scored by aid of experienced interventional cardiologists, without the knowledge of viral status results.
Sampling
The study group were a random sample of sixty-one HCVseropositive patients Presented to Ain Shams catheterization lab for coronary angiography
Data management
Data was collected, summarized and reported on data collection sheets. Data was reentered into computer Microsoft Excel sheets with appropriate tabulation and graphical presentation by SPSS version 22.
Statistical analysis:
All data was analyzed using:
Chi square was used to compare different frequencies.
Description of quantitative variables in the form of mean and standard variation.
Chi square was used to compare different frequencies.
Description of qualitative variables in the form of frequency and percentages.
T-student test of independent samples was used to compare each two quantitative groups.
Correlation Co-efficient test (r-test) was used to rank the different variables against each other either directly or indirectly.
X2 = Chi - square test was used to compare quantitative variables.
Significance level (P) value:
o P>0.05 = insignificance test
o P<0.05 = significance test
o P<0.001 = highly significance test.
Results
Descriptive analysis of data
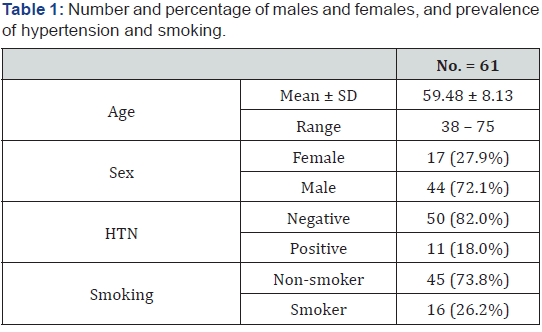
Patients demographics and medical history Table 1.
Mean age of patients in this study was 59.48 ± 8.13 and ranges from 38 to75 years. Most patients were males (72.1%). Most patients in the study were non-hypertensive 82%, with prevalence of non-smokers 73.8%.
Among patients included in the study 75% presented by chest pain, and 62% of patients had history of breathlessness
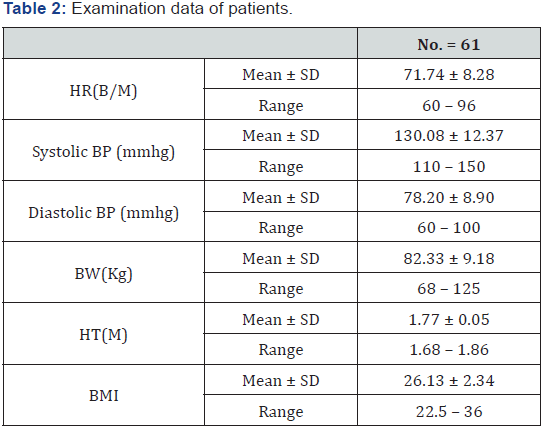
Examination data of study group
The mean body mass index of patients was 26.13 ± 2.34 (Table 2).
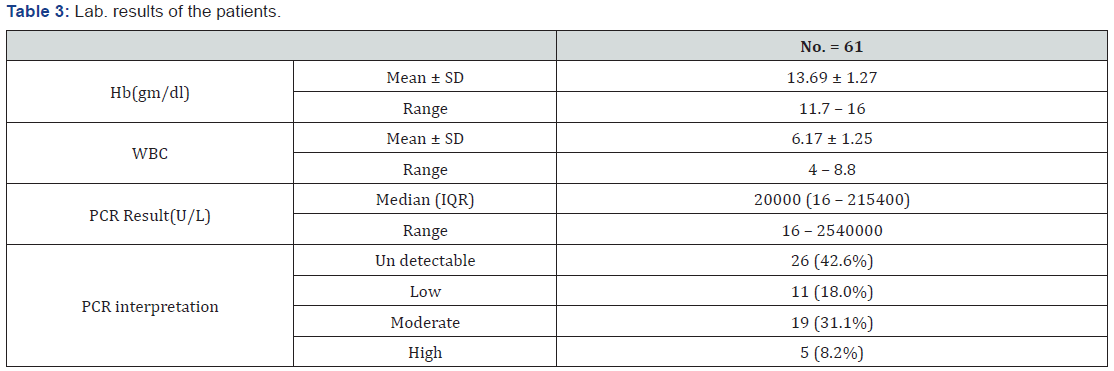
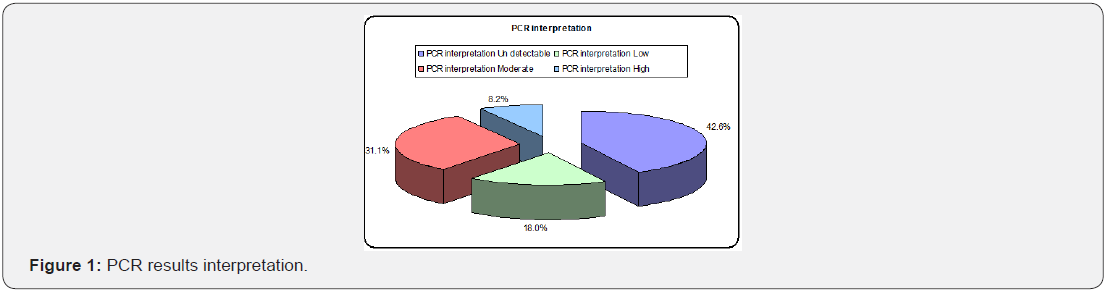
Laboratory data of the patients
As shown in (Table 3). And (Figure 1).
Angiographic data of patients as shown in Table 4.
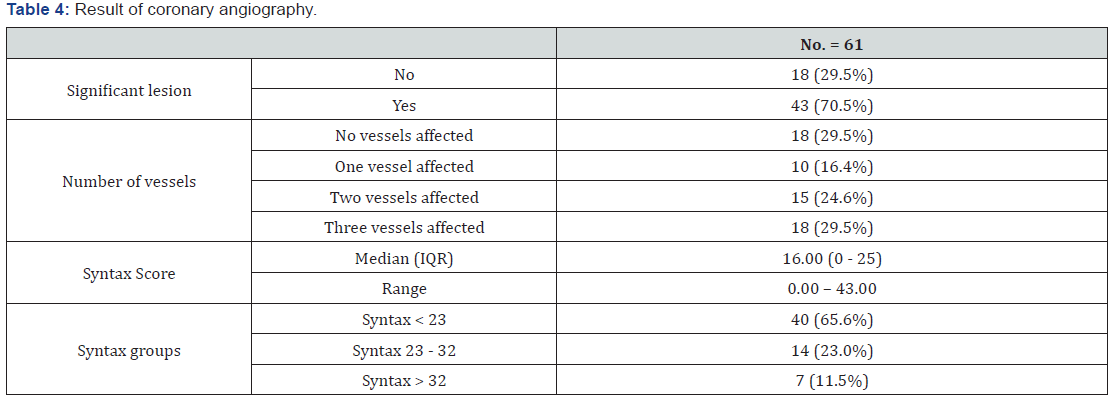
Patients who had three vessels disease were eighteen (29.5%), those with two vessels disease were fifteen (24.6%) and those who had one vessel disease were ten patients (16.4%) (Figure 2).
Comparative analysis of data
Correlation between PCR results and demographic data:
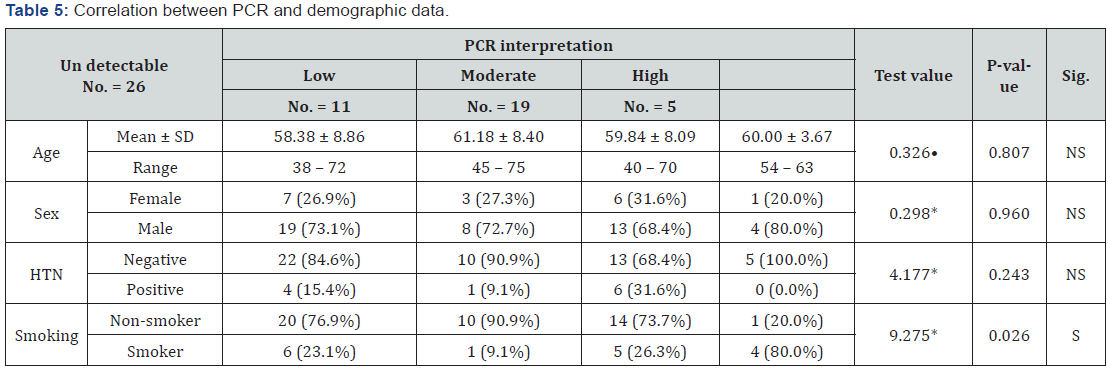
The PCR results was significantly correlated to smoking, (Table 5).
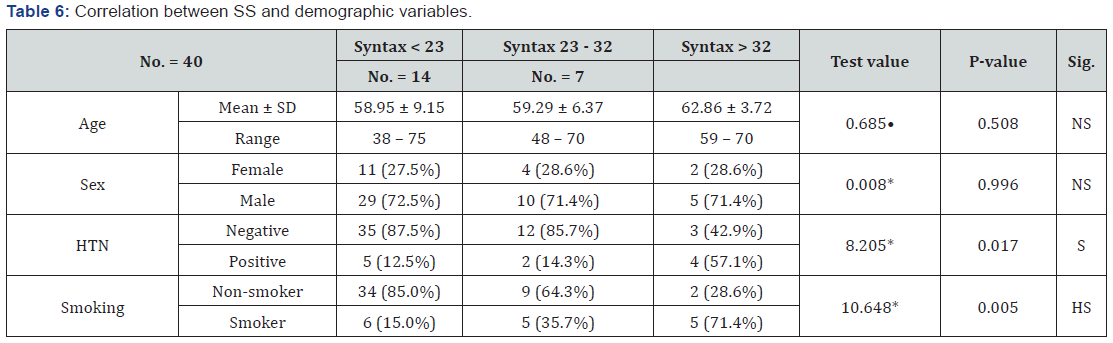
Correlation between Syntax score and demographic variables
Syntax score was significantly correlated to the presence of hypertension and highly correlated to smoking. Table 6.
Correlation between angiographic data and viral load as assessed by PCR Table 7 and Figure 3-5.
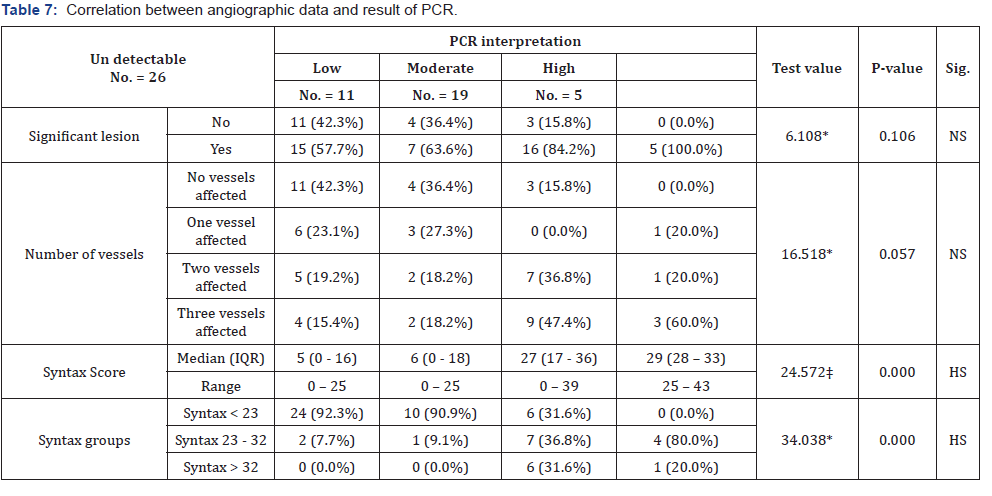


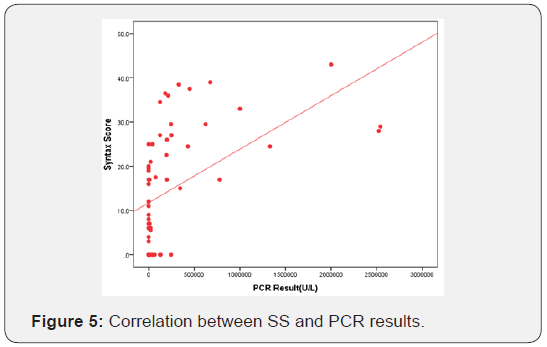
PCR level was highly correlated to the absolute SS and to the SS group, yet it was not found to be significantly correlated to the presence of significant coronary lesion nor to the number of vessels affected. On the other hand, SS was highly correlated to the presence of significant coronary lesion and to the number of vessels affected.
Discussion
This prospective cohort study was conducted to determine how Hepatitis C and its severity assessed by PCR influence disease progression in persons with coronary artery disease being assessed by Syntax Score. The study was carried out in the Cardiovascular Diseases Unit at Ain Shams University hospitals, from November 2015 until September 2017
The severity of CAD as assessed by Syntax score and the level of HCV RNA as detected by quantitative PCR were compared among sixty-one patients with positive HCV antibody test attending at Ain Shams University hospitals for elective coronary angiography.
Mean age of patients enrolled in the present study was 59.48 ± 8.13 and it ranged from 38 to75 years. Most patients were males (72.1%).
Several studies have evaluated the association between HCV and CAD. CAD was defined variably among the studies.
Vassalle et al. [5] first investigated the association between HCV infection and Coronary Artery Disease (CAD) in a case control study of 491 patients with angiographic documentation of CAD (stenosis >50%) and a control group of 195 patients admitted to the same institute for reasons other than suspected CAD, the mean age of study population was 66, 81.3% of them were males. The authors assessed the prevalence of HCV seropositivity in CAD patient’s vs controls [5].
Momiyama et al. [15] compared the prevalence of HCV antibody positivity among angiographically documented CAD (at least 50% stenosis in a major coronary artery)524 patients and106 as controls, with mean age of 64, and 84% of them were males [15].
Another case control stud by Arcari et al. [16] compared the prevalence of HCV infection between 292 MI patients vs. control group of 290 subjects, the study included young, active-duty USA military personnel, all subjects were males, and 60.6% of the study population were white, 30.1% were black, and 9.25% for others, the mean age was 42.2 years [16].
Another study conducted in Turkey between 2003 and 2007 by Alyan et al. [17] enrolled 364 CAD patients (139 HCV antibody positive patients VS. 225HCV negative patients as control), the mean age of study population was 61.2 years and 76.3% were males. All patient underwent coronary angiography and the rate of multi-vessel disease and Reardon severity score were compared in HCV antibody positive patients VS. uninfected control group, after adjustment for age, sex, smoking, hypertension, DM, Body Mass Index (BMI), CRP and fibrinogen [17].
In a US retrospective cohort of 171665 subjects (HCV infected: 82089, and controls: 89582), with mean age of 51.2 years and 97.2% were males, White constitute 55.4%,29.5 were black and Hispanic were 1.9 Butt et al assessed the risk of CAD in both group from 2001 to2006 after adjusting traditional risk factors [18].
A community based long term prospective study in a cohort of 23820 Taiwanese adults, enrolled from 1991 to 1992, and with follow-up data up to 2008, the study investigated all causes of mortality in anti-HCV positives compared with anti-HCV negatives [19]. In a large a retrospective cohort study from the United States of America (USA), Pothineni et al. [20] enrolled 24484 patients who further subdivided into three groups(HCV antibody positive: 8251, HCV RNA positive: 1434, and a controls: 14799), the mean age of the study population was 47.3 year for HCV antibody positive group and 48.6 for HCV-RNA positive group, and the percentage of male was 56.3% and 57% in both groups respectively, white Americans constitute about 77%, African Americans were18.5%, the incidence of CAD compared among these three groups [20].
Most patients in the present study were non- hypertensive 82%, with prevalence of non-smokers (73.8%). Among patients included in the study 75% presented by chest pain, and 62% of patients had history of breathlessness.
The mean body mass index of patients was 26.13 ± 2.34. More than two third of patients had significant lesion(s)on coronary angiography (70.5%). Patients who had three vessels disease were eighteen (29.5%), those with two vessels disease were fifteen (24.6%) and those who had one vessel disease were ten patients (16.4%). The present study revealed that PCR results was significantly correlated to smoking, it also showed significantly correlation between Syntax score and the presence of hypertension and smoking. On the other hand, SS was highly correlated to the presence of significant coronary lesion and to the number of vessels affected.
The most important finding of the present study was that PCR level (viral load) was highly correlated to the absolute SS and to the SS group, yet it was not found to be significantly correlated to the presence of significant coronary lesion nor to the number of vessels affected.
The present study showed significant relation between HCV RNA and CAD being assessed by SS and that patients with detectable HCV RNA had a significantly higher SS compared to those with only HCV antibody positive but no detectable RNA.
These findings agree with the results of many studies investigated the possible association between HCV infection and CAD, as that by Vassalle et al. [5], which reported a significant higher prevalence of HCV seropositivity in the CAD group compared with controls (6.3% vs 2.0%) [5].
Similar results reported by Alyan et al. [17] which demonstrated that HCV infection was an independent predictor of severity of coronary atherosclerosis, and that HCV infected patients had higher serum levels of both C reactive protein and fibrinogen, and a higher Reardon severity score compared with uninfected patients [17].
In the study by Butt et al. [18], the authors reported a significantly higher risk of incidental CAD in HCV infected subjects compared with those who were uninfected, despite the better metabolic profile reported in the first group and furthermore; HCV infection may independently predict an increased severity of CHD [18]. Interestingly, Lee et al. [19], reported that anti-HCV positives had a higher mortality from both hepatic and extrahepatic diseases compared with anti-HCV negatives, showing an adjusted HR of 1.50 for circulatory diseases [19].
The authors highlighted that this increase in mortality from circulatory diseases was maintained in anti-HCV patients with detectable HCV RNA, but not in those with undetectable HCVRNA.
The study by Pothineni et al. [20], the authors depended not only on HCV antibody positivity but also on measuring HCV RNA to investigate the association between HCV infection and CHD, the study showed that HCV antibody positivity (p < 0.001) and HCV RNA positivity (p < 0.001) were independent risk factors for CHD events and that patients with detectable HCV RNA had a significantly higher incidence of CHD events compared to those with only HCV antibody positive but no detectable RNA (5.9% vs. 4.7%, p = 0.04) [20].
A meta-analysis of ten studies by Olubamwo et al. [21] concluded that HCV infection may increase the risk of occurrence and the severity of coronary atherosclerosis (assessed by Gensini score), which seems consistent with the results of the vast majority of studies evaluating the effect of HCV infection on severity of CAD [21].
On the other hand, some studies found no significant correlation between HCV infection and coronary atherosclerosis, as the study by Arcari et al. [16] in which the authors found no association between HCV and AMI. This can be attributed to the fact that most MI occurs in relation to nonsignificant coronary lesions and to the different pathology of MI from that of SCAD.
Similarly, Momiyama et al. [15] reported comparable rates of HCV antibody positivity among angiographically documented CAD (at least 50% stenosis in a major coronary artery) patients and controls [15].
However, there are great differences between these two studies which found no significant correlation between HCV infection and coronary atherosclerosis, and the other studies showed evidence about role of HCV infection in the development of CAD and its severity including the present study
One difference about Arcari et al. [16] that the study was carried out on young active-duty military personnel with mean age of 42.2 who had recent AMI, and all patients were males. Also, the study periods were different, which may have reflected changing HCV treatment options since the regimen of pegylatedinterferon (IFN) alpha and ribavirin was approved in 2002 [16].
Another reason for discrepancies in results of different studies may be attributed to varying demographics of patients included in each study, and different genotype of the HCV infection.
The way of determination of HCV status (by HCV antibody or by HCV RNA) also may affect the results.
Conclusion and Recommendations
We concluded that the level of HCV RNA detected by quantitative PCR was correlated to the extent and severity of coronary artery disease as assessed by Syntax score in HCV antibody positive patients who were evaluated for CAD by angiogram. And that the higher the viral load in HCV seropositive patients, suggest greater severity of CHD. Yet it was not found to be significantly correlated to the number of vessels affected.
HCV RNA level predicts future cardiovascular risk, the incidence and severity of CAD is much higher in patients with detectable HCV RNA compared with patients with remote infection who are only HCV antibody positive with undetectable HCV RNA.
Chronic hepatitis C virus infection should be considered a systemic disease rather than a simple infection of the liver, and further studies are needed to assess the correlation between CAD severity and HCV infection.
Chronic HCV infection is a risk factor for atherosclerosis, development of cardiovascular diseases and significant cardiovascular mortality. The pathogenic mechanisms should be further investigated, due to their potential impact on the development of novel therapeutic approaches to prevent and to treat cardiovascular complications in patients with chronic HCV infection.
Treatment for hepatitis C has advanced tremendously and it is now possible to achieve cure with the new medications, therefore further research is needed to assess the effect of cure on CAD severity regression and cardiovascular risk in these patients.
References
- Nobili V, Carter-Kent C, Feldstein AE (2011) The role of lifestyle changes in the management of chronic liver disease. BMC Med 9: 70.
- Moller S, Henriksen JH (2010) Cirrhotic cardiomyopathy. J Hepatol 53(1): 179-190.
- Zardi EM, Abbate A, Zardi DM, Dobrina A, Margiotta D, et al. (2010) Cirrhotic cardiomyopathy. J Am Coll Cardiol 56(7): 539-549.
- Ishizaka N, Ishizaka Y, Yamkado M (2014) Atherosclerosis as a possible extrahepatic manifestation of chronic hepatitis C virus infection. Clin Med Insights Cardiol 8(Suppl 3): 1-5.
- Vassalle C, Masini S, Bianchi F, Zucchelli GC (2004) Evidence for association between hepatitis C virus seropositivity and coronary artery disease. Heart 90(5): 565-566.
- Esmat G (2013) Hepatitis C in the Eastern Mediterranean Region. East Mediterr Health J 19(7): 587-588.
- Simmonds P, Holmes EC, Cha TA, Chan SW, McOmish F, et al. (1993) Classification of hepatitis C virus into six major genotypes and a series of subtypes by phylogenetic analysis of the NS-5 region. J Gen Virol 74(Pt 11): 2391-2399.
- Danesh J, Collins R, Peto R (1997) Chronic infections and coronary heart disease: is there a link? Lancet 350(9075): 430-436.
- Matsumori A, Shimada T, Chapman NM, Tracy SM, Mason JW (2006) Myocarditis and heart failure associated with hepatitis C virus infection. J Card Fail 12(4): 293-298.
- Ishizaka Y, Ishizaka N, Takahashi E, Unuma T, Tooda E, et al. (2003) Association between hepatitis C virus core protein and carotid atherosclerosis. Circ J 67(1): 26-30.
- Ndrepepa G, Tada T, Fusaro M, Cassese S, King L, et al. (2012) Association of coronary atherosclerotic burden with clinical presentation and prognosis in patients with stable and unstable coronary artery disease. Clin Res Cardiol 101(12): 1003-1011.
- Gokdeniz T, Kalaycioglu E, Aykan AC, Boyaci F, Turan T, et al. (2014) Value of coronary artery calcium score to predict severity or complexity of coronary artery disease. Arq Bras Cardiol 102(2): 120-127.
- European Association for Study of Liver (2014) EASL Clinical Practice Guidelines: management of hepatitis C virus infection. J Hepatol 60(2): 392-420.
- Gokdeniz T, Turan T, Aykan AC, Gul I, Boyaci F, et al. (2013) Relation of epicardial fat thickness and cardio-ankle vascular index to complexity of coronary artery disease in nondiabetic patients. Cardiology 124(1): 41-48.
- Momiyama Y, Ohmori R, Kato R, Taniguchi H, Nakamura H, et al. (2005) Lack of any association between persistent hepatitis B or C virus infection and coronary artery disease. Atherosclerosis 181(1): 211- 213.
- Arcari CM, Nelson KE, Netski DM, Nieto FJ, Gaydos CA (2006) No association between hepatitis C virus seropositivity and acute myocardial infarction. Clin Infect Dis 43(6): e53-56.
- Alyan O, Kacmaz F, Ozdemir O, Deveci B, Astan R, et al. (2008) Hepatitis C infection is associated with increased coronary artery atherosclerosis defined by modified Reardon severity score system. Circ J 72(12): 1960-1965.
- Butt AA, Xiaoqiang W, Budoff M, Leaf D, Kuller LH, et al. (2009) Hepatitis C virus infection and the risk of coronary disease. Clin Infect Dis 49(2): 225-232.
- Lee MH, Yang HI, Lu SN, Jen CL, You SL, et al. (2012) Chronic hepatitis C virus infection increases mortality from hepatic and extrahepatic diseases: a community-based long-term prospective study. J Infect Dis 206(4): 469-477.
- Pothineni NV, Delongchamp R, Vallurupalli S, Ding Z, Dai Y, et al. (2014) Impact of hepatitis C seropositivity on the risk of coronary heart disease events. Am J Cardiol 114(12): 1841-1845.
- Olubamwo OO, Aregbesola AO, Miettola J, Kauhanen J, Tuomainen TP (2016) Hepatitis C and risk of coronary atherosclerosis - A systematic review. Public Health 138: 12-25.






























