Arachidonic Acid Metabolism, Thrombosis, and Stroke
Gundu HR Rao*
Laboratory Medicine and Pathology, Lillehei Heart Institute, University of Minnesota, USA
Submission:May 05, 2018; Published: June 06, 2018
*Corresponding author: Gundu HR Rao, Emeritus Professor, Laboratory Medicine and Pathology, Director, Thrombosis Research, Lillehei Heart Institute, University of Minnesota, USA, Email: gundurao9@gmail.com
How to cite this article: Gundu H R. Arachidonic Acid Metabolism, Thrombosis, and Stroke. J Cardiol & Cardiovasc Ther. 2018; 11(1): 555803. DOI: 10.19080/JOCCT.2018.11.555803
Introduction
Platelet hyper function resulting in the initiation of a thrombotic state of the circulating blood promotes the development of acute vascular events such as heart attacks and stroke [1-6]. Platelets circulating in blood interact with a variety of soluble agonists, such as Adenosine diphosphate (ADP), Epinephrine, many insoluble cell matrix components, including fibronectin, collagen, laminin and biomaterials used for the construction of medical devices and implants. The platelets play a critical role in the recognition of vessel-wall injury, formation of effective hemostatic plugs, retraction of clots and wound healing. When hyperactive, they can initiate events leading to many clinical complications associated with acute cardiovascular and cerebrovascular events. In this overview, we will describe platelet activation mechanisms, role of arachidonic acid metabolites in activation and inactivation mechanisms, and discuss aspirin resistance and its clinical significance.
In the circulating blood, normally there will be between 150, 000 to 450,000 platelets per microliter. They circulate in their discoid form and can interact and form aggregates with the slightest stimulation. Shown below are electron micrographs of vascular surface covered with healthy endothelial cells (A), platelets getting activated on a surface and pseudopod formation (B), and platelet activation on a denuded rabbit aorta (C) (Figure 1A-1C). Circulating fibrinogen plays an important role in agonist mediated platelet aggregation. Although large quantities of fibrinogen circulate in blood, platelets do not interact with this protein until fibrinogen biding receptor glycoprotein 11/111α is activated. Agonist-mediated activation results in the activation of this receptor and recognition of the tripeptide-binding site (arginine-glycine-aspartic acid, RGD) on this molecule (fibrinogen). If fibrinogen is bound on the surface, then there is no need for the activation of GP11/111α receptor for the platelet interaction. Platelets can interact with a variety of cell matrix components such as collagen, fibronectin, and laminin. Common anti-platelet drugs do not prevent this interaction. Whereas, agonist induced activation, aggregation, and thrombus formation are prevented by most of the anti-platelet drugs.
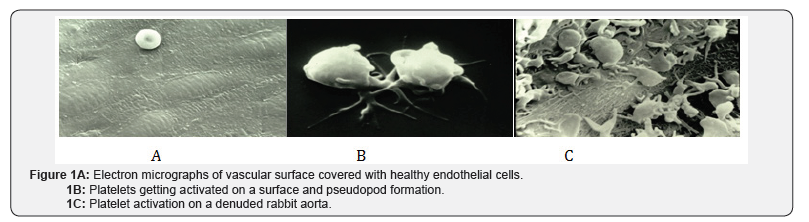
Platelets do not interact with a healthy endothelial surface (Figure 1A). Endothelial cells generate a variety of anti-aggregatory compounds such as Prostaglandin E1, Prostacyclin (PGI2), Adenosine, Thrombomodulin, proteins- S, protein- C, plasminogen activators, heparin-like glycosaminoglycans, and nitric oxide (NO). Agonist-induced activation at the platelet membrane receptor level, initiates a series of events associated with transmembrane signal transduction. If we consider thrombin as the stimulant, then it acts at the receptor site and releases thrombin receptor activating protein (TRAP), which initiates the transmembrane signaling via GTP coupled receptors (GTPR). Hydrolysis of GTP to GDP results in the activation of membrane associated Phospholipase C (Figure 1D) and hydrolysis of Phosphatidyl inositol 4, 5 bis- phosphate (PIP2) and formation of second messengers’ 1, 2-diacylglycerol (DG) and 1, 4, 5-inositol trisphosphate (IP3). Diglyceride activates protein kinase C (PKC), induces translocation of cytosolic PKC to membranes, whereas IP3 mobilizes calcium from the internal membrane stores. Elevated cytosolic calcium is essential for the assembly of soluble actin to filamentous form. Calcium plays a major role in contraction and secretion of granule contents.

Elevation of cytosolic calcium also activates phospholipase A2, which releases arachidonic acid from membrane associated phospholipids. This step is critical for the formation of prostanoids, which play a very important role in platelet activation and inactivation mechanisms (Figure 1E). Arachidonic acid (AA) is a 20-carbon polyunsaturated fatty acid and is the preferred substrate for the cyclooxygenase enzymes (COX-1) for the generation of prostaglandin (PG) endoperoxides. COX-1 enzymes convert AA into two transient bioactive molecules, PGG2 and PGH2. These endoperoxides are further transformed by thromboxane synthetase to Thromboxane A2 (TXA2) the major metabolite in platelets. Whereas in the vessel wall, these PG-endoperoxides, are converted by prostacyclin synthetase to prostacyclin (PGI2). Thus from a single substrate (AA) two major pharmacologically opposing molecules are generated.
Thromboxane is a potent agonist and vasoconstrictor. Prostacyclin is a potent antagonist and a vasodilator. Thromboxane A2 released by platelets activates circulating platelets via specific thromboxane receptors. Agonist induced-activation of platelets results in the elevation of cytosolic calcium, whereas, antagonists like prostacyclin, PGE1, PGD2, Nitric Oxide and adenosine, lower the elevated calcium levels by modulating the levels of cAMP or cGMP via respective enzymes, adenylyl cyclase and guanylyl cyclases. To a large extent, platelet activation and inactivation mechanisms are modulated by the elevation or lowering of cytosolic calcium.
Even before the prostaglandins were discovered, aspirin (acetyl salicylic acid) was in use as an anti-platelet drug. Once the prostaglandins were discovered studies done in several laboratories, established the specificity of this drug to COX- 1 enzymes. These studies further demonstrated that Aspirin acetylates hydroxyl groups of serine residue (Ser530) on the enzyme, and irreversibly inhibits the enzyme [7-9]. Since then hundreds of clinical trials have validated the beneficial effect of aspirin. Data for Aspirin as a drug of choice for the primary prevention strategies comes from the Antithrombotic Trialist’s Collaboration (ATT), which evaluated 95,456 patients in six clinical trials [10]. In the 2002 analyses by this group, 200,000 high-risk individuals in 287 studies were compared. Aspirin was the most widely studied anti-platelet drug. Indirect and direct comparisons suggested that doses 75-100mg were at least as effective as higher doses [11].
Aspirin resistance is a phenomenon that is poorly understood and results of several studies have added more confusion than clarity to explain this observation. Studies from our laboratory over three decades, have failed to show any aspirin resistance in normal healthy subjects. The only subject who failed to aggregate in response to arachidonate stimulation was found to be deficient in platelet COX-1 enzymes. In a classical study, we at the University of Minnesota demonstrated that if we create hindrance to the action of aspirin on the active site of COX-1 enzymes then inhibitory effect of aspirin of this enzyme could be prevented. In this study, we used a short acting drug Ibuprofen first and then followed with an additional dose of aspirin and showed that in the presence of Ibuprofen aspirin failed to irreversibly inhibit COX- 1 enzymes [12]. Because of the differences in the methodologies used to monitor the phenomenon of aspirin resistance and lack of specific assay to determine the true aspirin resistance, there is considerable confusion and the true significance of this remains obscure. Having said that, we want to inform the readers that there are studies, in which in spite of aspirin prophylaxis, increased levels of urinary thromboxane metabolites have been observed. In those cases it has been documented that such patients are at risk for developing acute vascular events.
Platelets play a very important role in the pathogenesis of atherosclerosis, thrombosis and stroke. Arachidonic acid metabolites, thromboxanes and prostacyclins play an important role in the modulation of platelet activation and inactivation mechanisms. Aspirin, even at a low to medium dose (80-120mg. day) has been shown to offer significant protection to individuals from developing acute vascular events. Aspirin is the most cost-effective drug available for the secondary prophylaxis of cardiovascular diseases. In view of these earlier observations, expectations of clinicians on the beneficial effect of aspirin therapy were very high. However, from the available evidence both experimental and clinical, it is reasonable to conclude, that significant number of patients are not getting full protection from the use of aspirin. Even those who are considered responders to aspirin therapy may need additional protection, if their platelets are hypersensitive or have a hyper-responsive coagulation pathway. Therefore, there are some limitations in the benefit derived from currently available anti-platelet therapy. Since currently available methods to assess aspirin sensitivity are not specific, labor intensive and time consuming; efforts should be made to develop specific rapid detection methods.
References
- Rao GHR (1999) Handbook of platelet physiology and pharmacology. Kluwer Academic Publishers, Boston, USA.
- Rao GHR, Kakkar VV (2001) Coronary artery disease in south Asians: epidemiology, risk factors and prevention. JP Medical Publishers, New Delhi, India.
- Rao GHR, Thanikachalam S (2005) Coronary artery disease: Risk promoters, pathophysiology, and prevention. JP Medical Publishers, New Delhi, India.
- Rao GHR (2016) Coronary artery disease. MacMillan Medical Communications (Springer/Nature) New Delhi, India.
- Rao GHR (1998) Platelets, prostaglandins and thrombosis. Med Update Assoc Phys Ind 8: 33-38.
- Rao GHR (2012) Aspirin resistance: clinical significance. J Clin Prevent Cardiol 1: 31-34.
- Lands WE (1979) The biosynthesis and metabolism of prostaglandins. Ann Rev Physiol 41: 633-652.
- Vane JR (1971) Inhibition of prostaglandin synthesis as mechanism of action of aspirin like drugs. Nature New Biol 231(25): 232-235.
- Roth J, Stanford N, Majerus PW (1975) Acetylation of prostaglandin synthase by aspirin. Proc Natl Acad Sci USA 72(8): 3073-3076.
- (1994) Collaborative overview of randomized trials of antiplatelet therapy 1: Prevention of myocardial infarction and stroke by prolonged antiplatelet therapy in various category patients, Antiplatelet Trialists Collaboration. BMJ 308(6921): 81-106.
- Baigent C, Blackwell L, Collins R, Emberson J, Godwin J, et al. (2009) Aspirin in the primary and secondary prevention of vascular disease: collaborative meta analysis of individual participant data from randomized trials. Lancet 373(9678): 1849-1860.
- Rao GH, Reddy KR, White JG, Johnson GG (1983) Ibuprofen protects platelet cyclooxygenase from irreversible inhibition by aspirin. Arteriosclerosis 3(4): 384-388.






























