P-Glycoprotein Expression in Heart, and its Potential Physiological Relevance
Alberto Lazarowski*
Department of Clinical Biochemistry, University of Buenos Aires, Argentina
Submission: April 09, 2018; Published: May 30, 2018
*Corresponding author: Alberto Lazarowski, PhD, Professor at Clinical Biochemistry Department, Institute of Physiopathology and Clinical Biochemistry (INFIBIOC), School of Pharmacy and Biochemistry, University of Buenos Aires (BA), Buenos Aires, Argentina, Email: nadiatom@ffyb.uba.ar
How to cite this article: Lazarowski A. P-Glycoprotein Expression in Heart, and its Potential Physiological Relevance. J Cardiol & Cardiovasc Ther. 2018; 10(5): 555799. DOI: 10.19080/JOCCT.2018.10.555799
Opinion
P-glycoprotein (P-gp) is the most studied member of ABC-transporters super-family. It is encoded by the MDR-1 gene (ABCB1), and share with BCRP (ABCG2) and MRP1-4 (ABCC1-4), the expression in the excretory organs and tissue barriers as liver, bowel, kidney, blood brain barrier, etc, playing a central protective role of the organism against endogenous/circulating chemicals, xenobiotics, toxins, organic ions and drugs. However, P-gp (as the others mentioned pumps) it is expressed neither in normal neurons nor in normal cardiomyocytes [1]. The nature of the expression of P-gp, indicates that it can be constitutive in mentioned tissues, as well as in stem cells [2], and also it can be induced to be expressed, after very wide spectrum of factors as different therapeutic agents (chemotherapeutic drugs), hormones, oncogenes, and transcription factors evolved in apoptosis, stress, inflammation, and hypoxia [3,4].
Early report demonstrated a variable expression of P-gp in heart (at the endothelium of both arterioles and capillaries, but not in cardiomyocytes) of all samples from 15 human left ventricles with non-failing (n=5), dilated cardiomyopathy (n=5), and ischemic cardiomyopathy (n=5), where the minor expression was observed in cases with dilated cardiomyopathy [5]. Later, in a chronically ischemic heart model in pigs, decreased 99mTc-SESTAMIBI heart retention was documented in the heart insulted area, where a high expression of P-gp can be observed in their cardiomyocites, as compared with the P-gp negative cells at the non-ischemic heart tissue [6]. In this experiment, the reason decreased 99mTc-SESTAMIBI heart retention supported by the low flow experimentally produced by chronic occlusion on the selected artery. Additionally, the high P-gp expression detected at cardiomyocytes in the same heart area could be supported by the hypoxic conditions that increase the expression of HIF-1 transcription factor as main driver for the inductor of P-gp expression.
Several techniques to assess the treatment-resistant phenotype in cancer, include demonstration of hypoperfusion with dynamic contrast-enhanced computed tomography and magnetic resonance imaging (MRI), depiction of necrosis with diffusion-weighted MRI, imaging of hypoxia and tumor adaption to hypoxia, and 99mTc-SESTAMIBI imaging of P-glycoprotein mediated drug resistance [7].
99mTc-SESTAMIBI is a cationic radiopharmaceutical that is widely used for evaluating cardiac function and for tumor imaging, in both cases the accumulation of this compound is known to occur within cells in response to the physiologically negative mitochondrial and plasma membrane potentials [8]. Interestingly, 99mTc-SESTAMIBI is substrate for P-gp and it can be effluxes in direct proportion to the cellular expression of P-gp [9]. This active washout is interpreted as chemoresistance phenotype of the tissue expressing P-gp. This functional phenotype can be inhibited by several P-gp and multidrug resistance modulators, such as PSC-833 and VX-710. Consequently, 99mTc-SESTAMIBI tumor washout can be used as clear biomarker for drug-resistant phenotype in tumors [10,11].
In bases on mentioned background: Which is the right interpretation for the low retention of 99mTc-SESTAMIBI in heart, observed in the cardiomyocytes of chronically ischemic pig hearts? Is it secondary to limited blood-flow, or secondary to HIF-1 induced P-gp expression exporting 99mTc-SESTAMIBI outside of the heart, or perhaps both mechanisms are contributing to the same result? The response to these controversial paradigms was later experimentally explained, in a model on conscious sheep undergoing acute myocardial ischemia followed by reperfusion. In this study similar decreased 99mTc-SESTAMIBI retention was documented by SPECT images and observed in all animals at the same ischemic-reperfused heart area. The i.v. administration of 99mTc-SESTAMIBI was performed three hours after reperfusion, the time that heart stunning was also documented, and P-gp expression was able to be detected in the cardiomyocytes of the same the ischemic heart area [12]. Some questions arise from these results.
a. Can a transient hypoxic condition to explain the decreased 99mTc-SESTAMIBI retention in heart without any artery occlusion and after the normoxia were restored?
b. Is the short time of artery occlusion (12 minutes) enough to prompt induction of functional P-gp expression?
c. Are there any relationship between the functional P-gp expression in cardiomyocytes and heart stunning?
One interesting condition could help to answer these questions, is the neurogenic stunned myocardium which is characterized by myocardial injury-dysfunction of a sudden onset, as a result of an imbalance in the autonomic nervous system. Sometimes it is secondary to different acute brain injuries as stroke, seizures, status epilepticus; panic attacks, etc. A spectrum of clinically abnormalities includes acute left ventricular failure could progress to cardiogenic shock with hypotension, pulmonary oedema and various arrhythmias, that include prolonged QT interval, ST segment changes, T-wave inversion, etc. [13]. Because the occurrence of neurogenic stunned myocardium can increases the death risk, several conditions related with high catecholamine’s release, has been suggested as potential inducers of these condition as pheochromacytoma and exogenous catecholamine administration. Furthermore, the Takotsubo Syndrome (TKS) was included into this spectrum of stress-related cardiomyopathies, where heart stunning was documented [14], and heart hypoxicischemia was postulated [15]. It was recently described that clinical presentation and pathophysiology of Takotsubo Syndrome differ from typical cardiomyopathies, and it was proposed that this syndrome must not be referred as a cardiomyopathy, it should be included within the spectrum of ischemic heart disease and considered as an acute syndrome, where the typical feature of stress, may imply that the catecholamine surge is essential to produce the typical transient myocardial injury [16].
It is known that TKS occurs after a stressful condition that including severe seizures and status epilepticus. Furthermore, after recompilation of several cases with TKS related to epileptic seizures, a possible relationship between TKS and sudden unexplained death in epilepsy (SUDEP) was suggested [17]. One more extensive analysis of this hypothesis suggest that sudden cardiac death can occur after exposure to extreme stress and sometimes as a complication of acute neurologic disease as severe seizures or status epilepticus. In this context, TKS could result from an excessive adrenergic stimulation of the heart that can also explain the sudden cardiac death. The sympathetic overstimulation could trigger a neurocardiogenic injury affecting the electrical properties of myocardium and leading to heart failure and fatal arrhythmia [18]. If it is the cases, TKS secondary to seizures, could be harbor a high risk of SUDEP. In more recent analysis of all reports of TKS related with epilepsy, it was detected that 60% of patients had generalized tonic clonic seizures, 32% had generalized status epilepticus, and only 3 cases had complex partial seizure, and fatal outcome was observed only in 2 cases (3%), suggesting that TKS does not seem to play a major role in the pathogenesis of SUDEP [19].
However, an important limitation of these considerations is the lack of opportunity to detect in real time, the TKS syndrome in patients that dead by SUDEP, so TKS could be an aborted- SUDEP. Sudden death was defined as the death that occurs naturally, unexpectedly and instantaneously or within the first hour of the onset of premonitory symptoms. It is one of the most important challenges of the modern cardiology and at date, several molecular studies have discovered different mutations related with the risk of sudden death. Recently were identified 4 major cardiac channelopathy genes mutations in KCNQ1, KCNH2, SCN5A, and RYR2 genes, which were classified as cardiac genetic predisposition in sudden infant death syndrome (SIDS) [20].
Sudden unexpected death in epilepsy (SUDEP) is the major cause of death in those patients suffering from refractory epilepsy (RE), with a 24-fold higher risk relative to the normal population. This catastrophic risk of epilepsy is not totally understood, and also is imprecisely detected. In spite several mutations on ion channels has been described to be related with - epilepsy, the above mentioned genes were not listed in a complete recent revision of genetics of epilepsy and refractory epilepsy [21]. A panel of experts from American Academy of Neurology (AAN), developed a multicenter survey of the incidence of SUDEP in different epilepsy populations, indicating that SUDEP risk in children with epilepsy is 0.22/1,000 patient-years, while the SUDEP risk increases in adults in patients (mainly aged between 20 and 40 years) to 1.2/1,000 patient-years. Additionally, subtherapeutic levels or interruption of AEDs, are also an important risk factor of SUDEP [22]. By far the most important clinical risk factor is frequency of GTCS, but nocturnal seizures, early age at epilepsy onset (before the age of 16 years), male gender, and long duration of epilepsy (over 15 years) have been identified as additional risk factors. If the seizure frequency is higher, the risk of SUDEP is greater. It is important to remark that refractory epileptic (RE) patients usually receive politherapy, but develop a multi-drug resistant phenotype due to brain overexpression of ABC-transporters, mainly P-glycoprotein, which was signed as potential therapeutic target y RE [23,24].
In spite that authors indicates that there is considerable uncertainty regarding the estimates of the real adult risk, the higher value of risk rate in adults, will not decrease with future more complete studies. So, in the clinical setting, the incidence of SUDEP in epileptic children is truly rare. Taking together, and in spite that mutations above mentioned are clearly related with SIDS, they could not be directly associated with SUDEP according with the epidemiological data described in the study from the AAN. An additional important recommendation also described in this mentioned AAN study, is that the major risk factor for SUDEP is the occurrence of generalized tonic-clonic seizures (GTCS), and it increase more secondary to the increasing frequency of GTCS. Which is the physiological meaning of this assertion? A very recent report describes a young woman with postictal apnea and generalized EEG suppression after a GTCS that was accompanied by severe bradycardia. The episode was followed by a ventricular tachycardia with subsequent recovery of spontaneous breathing, without any cardio-respiratory resuscitation maneuver [25].
However, the sudden and unexpected death in epilepsy (SUDEP) is a high challenge for the neurology, epileptogoly and cardiology. Some reports identified ion-channels mutations that could links between epileptic syndromes with Brugada Syndrome [26]. One very complete multicentric study, can detect mutations in genes related with cardiac arrhythmia, respiratory control, and epilepsy, in 46% of 61 SUDEP cases. The study was developed by complete exome sequencing compared with 2,936 control exomes, and in an additional focus was putted on cardiac arrhythmia, respiratory control, and epilepsy genes which were screened for variants with frequency of < 0.1% and predicted to be pathogenic with multiple in silico tools. Positive results were detected in 28 of 61 (46%) cases, and divided in three different categories defined a as: de novo mutations, previously reported pathogenic mutations, or candidate pathogenic variants. In four cases LQTS gene mutations were detected and in which SUDEP may occur as a result of a predictable and preventable cause [27].
Is this study covering completely all the genetic possibilities that could explain the phenomenon SUDEP? Which could be the genetic marker for the remaining 54% of the studied cases? Are there differences between germ line and somatic mutations as recently reported in atrial fibrillation an abnormal condition also described in SUDEP? [28,29]. According with these observations some electric heart alterations are commonly found during or after seizure, particularly in patients with refractory epilepsy, and they can offer a key answers for our questions [30-32]. One alternative mechanism can be the presence of a previously absent protagonist in this stressing scenario. So, transcriptome unbalance, with both up and down-regulation of several genes, could change the rules of the game in the functionality of cells, particularly in their pro or antiapoptotic tools, as well as their membrane electrical properties. In this regard, stress-induced expression in heart of genes related to cell salvage against the danger of oxidative stress, nutrient demand or hypoxia, could play a central roles in changing the these functional activity of the organ, but trying to save the cells.
HIF-1α is the master transcriptional regulator of cellular and developmental response to hypoxia in all tissues, and seizures induce hypoxic-ischemic events, nor only in brain but also in the systemic level, depending on their severity, duration and/or frequency. Our group can demonstrate a simultaneous induction of P-glycoprotein over expression in brain (neurons) and heart (cardiomyocytes), in a model of repetitive induced-seizures, and related with pharmacorresistant epilepsy and final fatal status epilecticus [33]. (Figure 1) We need to remember that P-gp is normally absent in these type of cells, and we must to think that these induced expressions are not only related to its pharmacoresistant property. At this - regards we should point out that pioneer studies have demonstrated that P-gp can modify the resting membrane potential, producing depolarization with values from -70 to -10mV in the expressing cells [34,35]. This specific observations is in total concordance with the mechanistic property of 99mTc-SESTAMIBI, that bind to mitochondrial membrane but it is released out off the cell by P-gp under membrane depolarization as above mentioned [8].
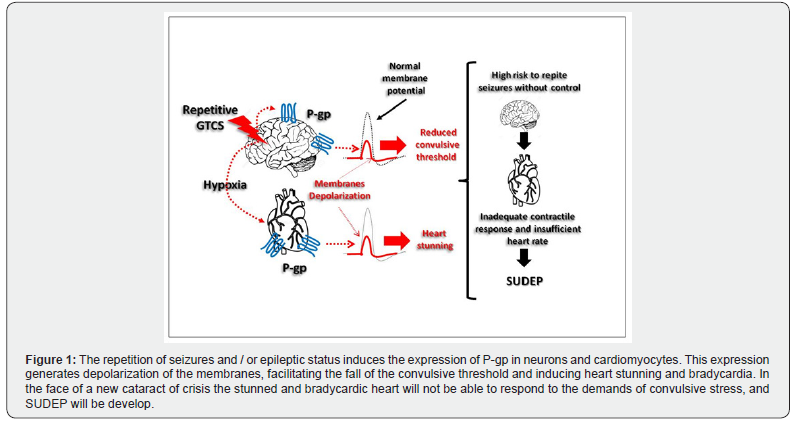
In these sense, P-gp expressed in neurons could be responsible at least in part, for the reduction of the convulsive threshold and facilitating the recurrence of new crises. In an our previous study, we demonstrated that increased expression of P-gp in hippocampus after repetitive induced-seizures, correlated progressive membrane depolarization that was not reversed by phenytoin, but it was do it by phenytoin plus a P-gp blocker nimodipine [36]. More recently, our group also demonstrated that repetitive sever convulsive stress as pilocarpine-induced status epilepcticus, resulted in a progressive high expression of HIF-1 and P-gp in cardiomyocites, detected during a period away from the acute convulsive episode, and accompanied by a severe bradycardia, longer QT interval, and high rate of spontaneous death. Interestingly, a low retention of 99mTc-SESTAMIBI in heart was also detected [37]. We could hypothesize that a fatal acute heart rhythm alteration could be the consequence of a severe hypoxic stress produced by repetitive seizures, where P-gp plays a depolarizing role on the cardiomyocytes. The potential use of non-invasive technologies as 99mTc-SESTAMIBI cardio-SPECT at both rest and effort conditions, could help to detect the risk of acute cardiological failure, and so, to be the telltale heart (EA Poe) for SUDEP in patients with refractory epilepsy.
References
- Cordon-Cardo C, O’Brien JP, Boccia J, Casals D, Bertino JR, et al. (1990) Expression of the multidrug resistance gene product (P-glycoprotein) in human normal and tumor tissues. J Histochem Cytochem 138(9): 1277-1287.
- Bunting KD (2002) ABC transporters as phenotypic markers and functional regulators of stem cells. Stem Cells 20(1): 11-20.
- Dean M (2005) The genetics of ATP-binding cassette transporters. Methods Enzymol 400: 409-429.
- Comerford K, Wallace T, Karhausen J, Louis N, Montalto M, et al. (2002) Hypoxia-inducible factor-1-dependent regulation of the multidrug resistance (MDR1) gene. Cancer Res 62(12): 3387-3394.
- Meissner K, Sperker B, Karsten C, Meyer zu Schwabedissen H, Seeland U, et al. (2002) Expression and localization of p-glycoprotein in human heart: effects of cardiomyopathy. J Histochem Cytochem 50(10): 1351- 1356.
- Lazarowski AJ, García Rivello HJ, Vera Janavel GL, Cuniberti LA, Cabeza Meckert PM, et al. (2005) Cardiomyocytes of chronically ischemic pig hearts express the MDR-1 gene-encoded P-glycoprotein. J Histochem Cytochem 53(7): 845-850.
- Kyle SD, Law WP, Miles KA (2013) Predicting tumour response. Cancer Imaging 13(3): 381-390.
- Piwnica-Worms D, Kronauge JF, Chiu ML (1990) Uptake and retention of hexakis (2-methoxyisobutyl isonitrile) technetium (I) in cultured chick myocardial cells: mitochondrial and plasma membrane potential dependence. Circulation 82(5): 1826-1838.
- Piwnica-Worms D, Rao V, Kronauge J, Croop J (1995) Characterization of multidrug resistance P-glycoprotein transport function with an organotechnetium cation. Biochemistry 34(38): 12210-12220.
- Sun SS, Hsieh JF, Tsai SC, Ho YJ, Lee JK, et al. (2000) Expression of mediated P-glycoprotein multidrug resistance related to Tc-99m MIBI scintimammography results. Cancer Lett 153(1): 95-100.
- Lazarowski A, Dupont J, Fernández J, Garay G, Florín A, et al. (2006) 99mTechnetium-SESTAMIBI uptake in malignant lymphomas. Correlation with chemotherapy response. Lymphat Res Biol 4(1): 23- 28.
- Laguens RP, Lazarowski AJ, Cuniberti LA, Vera Janavel GL, Cabeza Meckert PM, et al. (2007) Expression of the MDR-1 gene-encoded P-glycoprotein in cardiomyocytes of conscious sheep undergoing acute myocardial ischemia followed by reperfusion. J Histochem Cytochem 55(2): 191-197
- Biso S, Wongrakpanich S, Agrawal A, Yadlapati S, Kishlyansky M, et al. (2017) A review of neurogenic stunned myocardium. Cardiovasc Psychiatry Neurol 2017: 5842182.
- Ancona F, Bertoldi LF, Ruggieri F, Cerri M, Magnoni M, et al. (2016) Takotsubo cardiomyopathy and neurogenic stunned myocardium: similar albeit different. Eur Heart J 37(37): 2830-2832.
- Ghadri JR, Bataisou RD, Diekmann J, Lüscher TF, Templin C (2015) First case of atypical takotsubo cardiomyopathy in a bilateral lungtransplanted patient due to acute respiratory failure. Eur Heart J Acute Cardiovasc Care 4(5): 482-485.
- Pelliccia F, Sinagra G, Elliott P, Parodi G, Basso C, et al. (2018) Takotsubo is not a cardiomyopathy. Int J Cardiol 254: 250-253.
- Dupuis M, van Rijckevorsel K, Evrard F, Dubuisson N, Dupuis F, et al. (2012) Takotsubo syndrome (TKS): A possible mechanism of sudden unexplained death in epilepsy (SUDEP). Seizure 21(1): 51-54.
- Rabinstein AA (2014) Sudden cardiac death. Handb Clin Neurol 119: 19-24.
- Finsterer J, Bersano A (2015) Seizure-triggered Takotsubo syndrome rarely causes SUDEP. Seizure 31: 84-87.
- Tester DJ, Wong LCH, Chanana P, Jaye A, Evans JM, et al. (2018) Cardiac genetic predisposition in sudden infant death syndrome. J Am Coll Cardiol 71(11): 1217-1227.
- Lazarowski A, Czornyj L (2013) Genetics of epilepsy and refractory epilepsy. In: Dean M (Ed.), Colloquium Series on the Genetic Basis of Human Disease. Morgan & Claypool Life Sciences, USA.
- Harden C, Tomson T, Gloss D, Buchhalter J, Cross JH, et al. (2017) Practice guideline summary: sudden unexpected death in epilepsy incidence rates and risk factors: report of the guideline development, dissemination, and implementation subcommittee of the American academy of neurology and the American epilepsy society. Epilepsy Curr 88(17): 1674-1680.
- Robey RW, Lazarowski A, Bates SE (2008) P-glycoprotein--a clinical target in drug-refractory epilepsy? Mol Pharmacol 73(5): 1343-1346.
- Lazarowski A, Czornyj L, Lubienieki F, Girardi E, Vazquez S, et al. (2007) ABC transporters during epilepsy and mechanisms underlying multidrug resistance in refractory epilepsy. Epilepsia 48(5): 140-149.
- Jin L, Zhang Y, Wang XL, Zhang WJ, Liu YH, et al. (2017) Postictal apnea as an important mechanism for SUDEP: A near-SUDEP with continuous EEG-ECG-EMG recording. J Clin Neurosci 43: 130-132.
- Sonoda K, Ohno S, Ozawa J, Hayano M, Hattori T, et al. (2018) Copy number variations of SCN5A in Brugada Syndrome. SCN5A CNVs in BrS. Heart Rhythm pii: S1547-5271(18)30254-6.
- Bagnall RD, Crompton DE, Petrovski S, Lam L, Cutmore C, et al. (2016) Exome-based analysis of cardiac arrhythmia, respiratory control, and epilepsy genes in sudden unexpected death in epilepsy. Ann Neurol 79(4): 522-534.
- Desai R, Rupareliya C, Patel U, Naqvi S, Patel S, et al. (2017) Burden of arrhythmias in epilepsy patients: a nationwide inpatient analysis of 1.4 million hospitalizations in the United States. Cureus 9(8): e1550.
- McCauley M, Darbar D (2017) Germline versus somatic mutations in genetic atrial fibrillation. Heart Rhythm 14(10): 1539-1540.
- Vedovello M, Baldacci F, Nuti A, Cipriani G, Ulivi M, et al. (2012) Periictal prolonged atrial fibrillation after generalized seizures: description of a case and etiopathological considerations. Epilepsy Behav 23(3): 377-378.
- Sanchez-Larsen A, Aznar-Lain G, Benito B, Principe A, Ley M, et al. (2017) Post-ictal atrial fibrillation detected during video-EEG monitoring: Case report, proposed physiopathologic mechanism and therapeutic considerations. Epilepsy Behav Case Rep 8: 40-43.
- Nei M, Ho RT, Sperling MR (2000) EKG abnormalities during partial seizures in refractory epilepsy. Epilepsia 41(5): 542-548.
- Auzmendi J, Merelli A, Girardi E, Orozco-Suarez S, Rocha L, et al. (2014) Progressive heart P-glycoprotein (P-gp) over expression after experimental repetitive seizures (ERS) associated with fatal status epilepticus (FSE). Is it related with SUDEP? Mol & Cell Epilepsy 1(3): 1-9.
- Wadkins RM, Roepe PD (1997) Biophysical aspects of P-glycoproteinmediated multidrug resistance. Int Rev Cytol 171: 121-165.
- Roepe PD (2000) What is the precise role of human MDR 1 protein in chemotherapeutic drug resistance? Curr Pharm Des 6(3): 241-260.
- Auzmendi JA, Orozco-Suárez S, Bañuelos-Cabrera I, González-Trujano ME, Calixto González E, et al. (2013) P-glycoprotein contributes to cell membrane depolarization of hippocampus and neocortex in a model of repetitive seizures induced by pentylenetetrazole in rats. Curr Pharm Des 19(38): 6732-6738.
- Auzmendi J, Buchholz B, Salguero J, Cañellas C, Kelly J, et al. (2018) Pilocarpine-induced status epilepticus is associated with p-glycoprotein induction in cardiomyocytes, electrocardiographic changes, and sudden death. Pharmaceuticals (basel) 11(1).






























