The Use of Gas Discharge Visualization for Identifying Structured Peculiarities of Blood Components: Plasma, Platelets, and Erythrocytes
MA Stepovich1*, OM Maslennikova2, MN Shipko3, AL Sibirev3 and VV Chrishtop4
1Tsiolkovsky Kaluga State University, Russia
2Central State Medical Academy of Department of Presidential Affairs, Moscow, Russia
3Lenin Ivanovo State Power Engineering University, Russia
4Ivanovo State Medical Academy, Russia
Submission: January 23, 2018; Published: March 09, 2018
*Corresponding author: MA Stepovich, Tsiolkovsky Kaluga State University, 26 Stepan Razin Str., Kaluga, 248023, Russia, Email: om_shipko@mail.ru
How to cite this article: MA Stepovich, OM Maslennikova, MN Shipko, AL Sibirev, VV Chrishtop. The Use of Gas Discharge Visualization for Identifying Structured Peculiarities of Blood Components: Plasma, Platelets, and Erythrocytes. J Cardiol & Cardiovasc Ther 2018; 9(4): 555770. DOI: 10.19080/JOCCT.2018.09.555770.
Abstract
The article presents the results of the research into optoelectronic emission of blood and its components before and after oxidation, the features of the emission parameters describing the positional order of molecular bindings, as well as the features of intermolecular interactions before and after exposure to oxidative stress on whole blood and its components. The revealed changes can be the basis for determining the mechanism of oxidative and reduction processes occurring in associations of protein molecules of cardiovascular system under the influence of environmental factors or chemical effects. It is proven that the markers of oxidative stress on whole blood and its components must be related to the parameters of the angular and energy distribution of the emitted electrons.
Keywords: Emission; Gas discharge visualization; Oxidative stress; Positional order; Molecular associates; Bioelectrography
Introduction
Small-angle X-ray scattering and polarization optical analysis are the most common ways of detecting long-lived post-transitional modifications of amino acids within protein structures of organisms [1]. They are both rarely used in laboratory studies because of the complexity of the results obtained and the relatively high material costs. The paper presents the use of gas discharge visualization (GDV) for determining the positional order and geometry of an organism’s molecular bindings in the aqueous substance [2]. This method is based on the initiation and study of the processes of optoelectronic emission from the aqueous substance. It is widely used in clinical trials to assess the biological function and homeostasis that are limited by molecular, cellular, and organism interactions [3]. The mechanisms of optoelectronic emission are still not studied well enough despite the existence of clinical and laboratory evidence of comparability of data obtained in GDV and electrocardiography or electroencephalography [4]. A huge variety of protein molecules of the cellular and tissue structures forms a complex picture of the emission of electrons and its parameters are averaged. This complicates the interpretation of the changes in the structural state of proteins of blood components and their relationship with changes in the functioning of body systems [5]. Therefore, the study of mechanisms of influence of physical and chemical factors on the parameters of optoelectronic emission of the aqueous substance of an organism determined by its structural state is especially interesting.
The purpose of this work was studying the features of the change in the oxidative stress on the fractal structure and the energy of electrons reflecting the state of whole blood proteins, red cell mass (RCM), platelet-rich plasma, and plasma.
Material and Methods
For a scientific research, a blood sample was taken into a standard 4.5ml vacuum tube with 3.8% sodium citrate. The tubes were placed in an EBA 20 centrifuge (Hettich, Germany) and were centrifuged for 10 minutes at the speed of 3500rpm. After rotation, the blood was divided into 3 main components: plasma (top layer), platelet-rich plasma (middle layer), and red cell mass (lower layer). The studied blood fractions were extracted from the tube using a syringe needle. The erythrocytes were washed twice with physiological saline after being separated from plasma. 0.5% hydrogen peroxide solution in a 5:1 ratio was added in order to simulate oxidative stress in a tube with whole blood, red cell mass, platelet-rich plasma, and blood plasma.
A GDV-device was used to study the processes of optoelectronic emission from a drop of whole blood or its components. The emission of electrons and photons was stimulated by bipolar electric pulses with an amplitude of 9kV with a duration of 3 microseconds, and a frequency 1024Hz. After being emitted from the surface and an inner part of the blood drop (8±0.07)*10- 9m3, electrons are accelerated by an electric field, generating electron avalanches. These avalanches cause ionization of the air and form a sliding gas discharge on the surface of the lens glass of the camera. The glow of the gas is converted into a digital code. It is later displayed on a computer screen as a gas-discharge image which is presented as spatially distributed glow sections with different brightness, length, and frequency. The parameters of the luminescence pattern are defined by the energy, amount, and angular distribution of electrons emitted from the blood drop and depending on the structural state of inner and outer parts of the drop, as well as the energy state of electrons that provide interaction between molecular bindings. The parametric analysis of the luminescence pattern, which is performed automatically using "GDV Scientific Laboratory" software, shows the details about the fractal structure, the shape coefficient of the studied blood drop, the degree of molecular bindings' ordering in the drop, etc. [2].
Results
Figure 1 shows averaged patterns of gas-discharge images of blood and its components before and after oxidation. There is a significant difference in the patterns of luminescence of plasma, platelets, and erythrocytes. In the initial state, blood is depicted as a shiny disk with radial traced of electron motion. These electrons are emitted from the surface and the inner part of the drop. The spatial arrangement and the number of streamers are not the same for different blood components. Considering that streamer directions are linked to the channels of facilitated motion of electrons in blood and its components, they indicate the direction of planes with the most dense packing of molecular bindings [6,7]. There are three directions in the initial state of plasma, four in the platelet-rich plasma, seven in the red cell mass, and six in whole blood.
After the exposure to oxidative stress, the picture of the glow of blood and its components has significant changes. The six directions in the blood are preserved after the modeling of oxidative stress. They differ in the parameters of the emitted electrons. After modeling of oxidative stress of blood plasma, the directions of electron motion in the volume and outside of the drop are the same. However, the width of the facilitated motion channels is significantly reduced (Figure 1). In case of platelets, the emission of electrons from the drop surface prevails, thus changing its shape (Figure 1). The picture of luminescence of red cell mass after its oxidation reproduces the gas-discharge image of the blood in the initial state. The observed changes in the gasdischarge picture of blood and its components are accompanied by changes in the luminescence parameters.

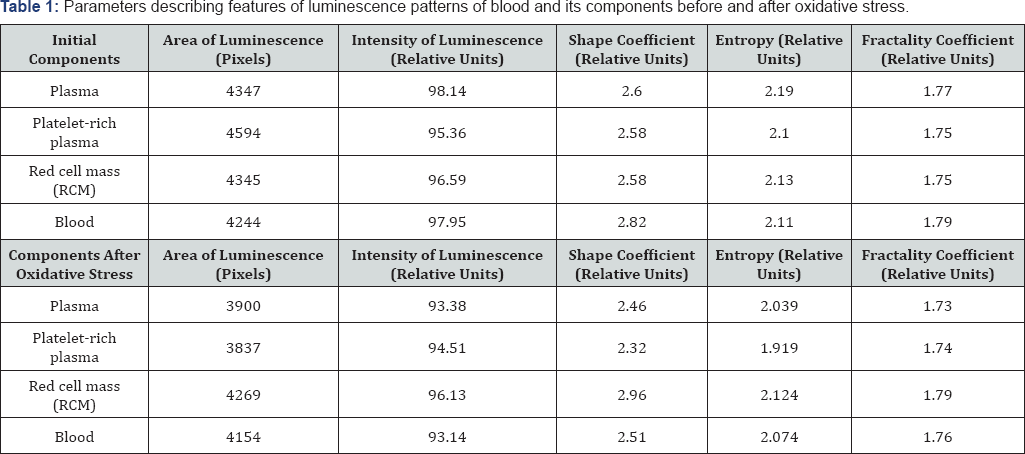
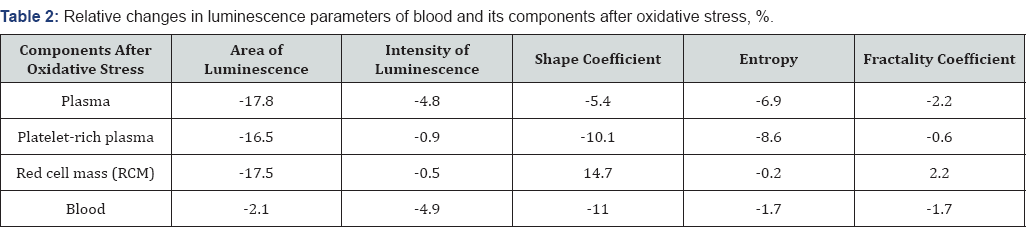
Table 1 shows the values of the parameters of luminescence patterns, which, by their modifications, reveal the features of the processes occurring in blood and its components during modeling of oxidative stress. Table 2 shows relative changes in those parameters after modeling of oxidative stress in blood and its components. The most noticeable changes after modeling of oxidative stress in the blood and its components can be seen in the area, the shape coefficient, and the luminescence entropy. Meanwhile, the most significant decrease in these parameters were observed for plasma, red cell mass, and platelets.
The luminescence area is determined by the amount and energy of the emitted electrons and, as a rule, represents the destructive features of the substance. These features are linked to a change in the spatial orientation of molecular bindings and interactions between them [7]. Based on data on the intensity and area of luminescence, our calculations indicate that the average energy of electrons emitted from the plasma increases by 6% and by 16% for the platelet-rich plasma.
In this case, the decrease in the luminescence area of blood components is associated with the decrease in the number of electrons emitted from an inner part of the drop. Due to the decrease in the values of the form factor and entropy, these changes can be justified by the changes in the positional order of molecular bindings and their size. The decrease in the entropy and form factor describing the spatial orientation of molecular bindings in the blood and its components indeed indicates a change in the positional order in the protein molecules arrangement after oxidation.
A decrease in entropy after oxidation of plasma and red cell mass, which is a measure of the system disorder, particularly justifies the decrease in the positional order of molecular bindings in the mentioned blood components [8]. At the same time, a decrease in the fractality coefficient, which is a measure of the geometric configuration of the positional order of molecular bindings, and the shape coefficient, which measures their size distribution, indicate changes in the spatial arrangement of these bindings.
Thus, the destructive processes taking place in the blood and its components during their oxidation can be explained by the changes in the parameters of optoelectronic emission. During oxidative stress, there is a disruption of the positional order in the molecular bindings' arrangement and, therefore, there is a change in the distribution of electrons during free-radical reactions. It is possible to assume the mechanism of both blood and the cardiovascular system diseases by the way of the electron energy distribution, their angular distribution during emission based on the revealed correlations between the emission parameters and the adaptive capabilities of the blood system [9,10]. In particular, the most significant changes in protein plasma molecules indicate a high availability of its proteins for peroxide reactions. The high sensitivity of platelets to oxidative stress, which provides a reduction in the emission parameters, is especially interesting. This indicates the formation of molecular cross links blocking surfaces of the platelets' certain sections [11].
Conclusion
The performed studies of blood and its components (plasma, platelet-rich plasma, and red cell mass) before and after oxidative stress using GDV show that the change in the parameters of optoelectronic emission of blood components after modeling of oxidative stress has the following reasons:
a) Changes in the positional order of molecular associates;
b) A decrease of the number of emitted electrons, that are linked to the change of the kind of intermolecular bonds inside and between associates, and an increase of their energy.
The obtained data can be used for evaluating the state of proteins of blood and its components, as well as the degree of biological structures damage under oxidative stress. Having all the data about the angular distribution of emitted electrons, the use of gas discharge visualization (GDV) can be used for revealing structural features of blood components when exposed to physical factors.
References
- Muehsam D, Chevalier G, Barsotti T, Gurfein BT (2015) An overview of biofield devices. Glob Adv Health Med 4(Suppl): 42-51.
- Korotkov KG (2007) GDV Principle Analysis, Renome, St. Petersburg, Russia, p. 286.
- Yakovleva EG (2013) The diagnostic possibilities of the GDV- bioelectrography. Method in medicine review. Vestnik of Medical Technologies (electronic edition) 1: 8.
- Shein AA, Khlebniy ES, Kershgolts BM (2004) Gas discharge visualization -perspectives of quantitative and qualitative determination of ingredients in liquid environment. Fundamental Research 7: 43.
- Korotkov KG, Matravers P, Orlov DV, Williams BO (2010) Application of Electrophoton Capture (EPC) Analysis Based on Gas Discharge Visualization (GDV) technique in medicine: a systematic review. J Altern Complement Med 16(1): 13-25.
- Shipko MN, Usoltseva NV, Sibirev AL, Maslennikova OM, Stepovich MA, et al. (2017) Magnetopulse effect on the structural state of surfactant solutions. Proc. XXV Int. Conf. electromagnetic field and materials (Fundamental Physical Research, Moscow Power Engineering University (National Research University), Russia, pp. 48-61.
- Shipko MN, Usoltseva NV, Sibirev AL, Maslennikova OM, Stepovich MA, et al. (2018) Influence of pulsed electromagnetic field on positional and orientational ordering in aqueous solution of cetyltrimethylammonium bromid (CTAB). Liq Cryst & Their Appl 18(1): 24-31.
- Opritov VA (1999) Entropy of Biosystems. Soros' Educational Magazine 6: 33-38.
- Kovelkova MN, Yakovleva EG, Korotkov KG, Belonosov SS, Zarubina TV (2014) Automated diagnostic system for detection of patient with arterial hypertension based gas discharge visualization technique. Diagnostic Systems 3: 28-34.
- Oschman JL (2007) Can electrons act as antioxidants? A review and commentary. J Altern Complement Med 13(9): 955-967.
- Maslennikov PV, Chupakhina GN, Krasnoperov AG (2013) The use of GDV in determination of the anti-oxide status of plants with toxic effects of cadmium. Vestnik of the Kant Baltic Federal University 7: 1427.






