HMGB1: A Therapeutic Target for Myocardial Infarction
Divya D, Arul Salomee KR, Anusuyadevi M and Jayachandran KS*
Department of Bioinformatics, Bharathidasan University, India
Submission: August 28, 2017; Published: August 31, 2017
*Corresponding author: Jayachandran KS, Assistant Professor, Drug Discovery and Molecular Cardiology Laboratory, Department of Bioinformatics, Bharathidasan University, Tiruchirappalli- 620024, India, Tel:+919840632789; Email: swaminathan.jayachandran@gmail.com/s.jayachandran@bdu.ac.in
How to cite this article: Divya D, Arul S K, Anusuyadevi M , Jayachandran KS. HMGB1: A Therapeutic Target for Myocardial Infarction. J Cardiol & Cardiovasc Ther 2017; 7(5): 555724. DOI: 10.19080/JOCCT.2017.07.555724
Abstract
Myocardial ischemia/reperfusion injury is a major socioeconomic problem worldwide. Though there are wider advancements, still mortality rate is substantial. This can be overcome by exploring complete pathopysiology of the disease mechanism. High-mobility group box-1 (HMGB1) is recently explored multifunctional protein based on its location. The present short communication gives an idea on multiple biological activities of HMGB1.
Introduction
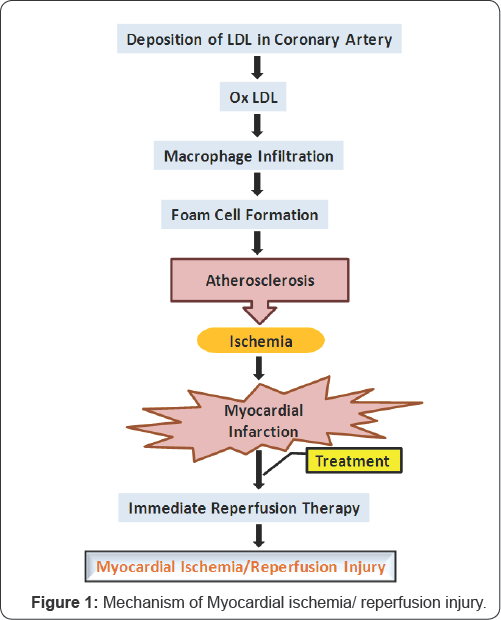
Myocardial infarction (MI) is the leading cause of death worldwide. Atherosclerotic plaque formation, blocks the coronary artery and there by leads to MI. Early reperfusion is required to salvage diseased myocardium. Reperfusion in turn damages the myocardium called as reperfusion injury (Figure 1). Myocardial ischemia/reperfusion (I/R) injury aggravates the area of infarction. Though there are several advancements in treatments, re-hospitalization and reoccurrence of MI is consistent. Translation of bench to bed side medicine is failed in several cases due to existence of multiple co-morbidities and co-medications; reproducing this in animal models is quite challenging [1]. New treatment strategies are required in order to overcome this problem. Inflammation and infiltration of immune cells are the hallmark in pathophysiology of MI. Inflammation acts as double edge sword with beneficial and detrimental effects. It elucidates body's reaction towards injury and infection. On one hand, inflammation helps in healing of injury and on the other hand, prolonged inflammation alters matrix integrity, cell survival and enhances inflammatory cytokines thereby shows deleterious effects and eventually leads to cardiac failure. Hence, anti-inflammatory strategies may reduce infarct size and protects myocardium.
Role of HMGB1
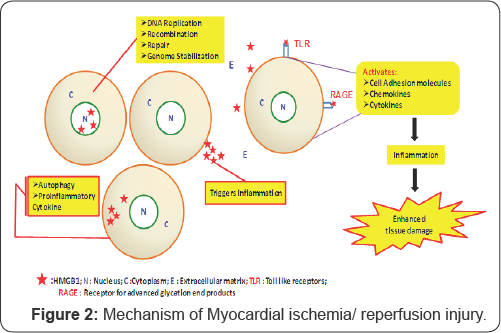
Multifaceted high-mobility group box-1 (HMGB1) proteins plays major role in cardiovascular diseases. HMGB1 is a highly conserved DNA-binding nuclear protein. Biological role of HMGB1 depends on cellular localization. Nuclear HMGB1 maintains chromosomal structure and stability. It plays a major role in DNA replication, repair, recombination and acts as transcriptional factor. HMGB1 is released into cytoplasm during cell injury and acts as cytokine there by activates inflammatory pathway. It acts as innate danger signal and amplifies inflammatory response during I/R injury [2]. In addition cytoplasmic HMGB1 activates autophagy on binding with Beclin 1 which initiates autophago some formation [3]. HMGB1 is released into extracellular matrix by necrotic cells and macrophages. HMGB1 belongs to "Damage Associated Molecular Pattern Molecules" that acts as alarmin during tissue damage and activates inflammatory and immune response [4]. Extracellular HMGB1 binds to toll like receptor 4 (TLR4) or receptor for advanced glycation end products (RAGE) and activates cytokines, chemokines and adhesion molecules there by triggers inflammatory process [3] (Figure 2).
Is HMGB1 Boon or Bane?
Studies proved that HMGB1 has detrimental effect on cardiac injury. Hence, it can acts as good diagnostic marker and therapeutic target. Kohno et al. have validated that increased expression of HMGB1 leads pump failure, cardiac rupture and death whereas, blockade of HMGB1worsened LV remodeling [5]. Andrassy et al. proved that HMGB1 activate proinflammatory pathways and enhance myocardial injury during MI [6]. Interestingly, in controversial to this Kitahara et al. [7] demonstrated that transgenic mice with cardiac specific HMGB1 over expression showed reduced infarct size, increased angiogenesis and survival rate after induction of MI when compared to wild type [7]. Limana et al. [8] stated that injection of HMGB1 into left ventricular wall of failing heart in murine model has increased LV function and animal survival by increased number of c-kit+ cells in the infarcted area [8].
Conclusion and Perspectives
Discovery of HMGB1 has opened new avenues in pathology of inflammatory diseases. Our understanding has revealed that role of HMGB1 depends on localization, level and point of expression, post translational modifications and receptor binding. Release of HMGB1 during early phase of injury might have beneficial role in wound healing and regeneration. On contrary, chronic accumulation of HMGB1 in extracellular matrix may trigger inflammation and there by leads to tissue damage. Multiple factors are involved in injury and healing process. HMGB1 have dual effects based on its modulation. To unravel the mechanism of bidirectional function of HMGB1further research is needed to understand molecular basis, deleterious and beneficial role of HMGB1 in cardiovascular diseases.
Sources of Funding
This work was financially supported by Food and Nutrition project, Department of Biotechnology, India: Grant BT/PR6327/ FNS/20/606/2012.
References
- Candilio L, Hausenloy D (2017) Is there a role for ischaemic conditioning in cardiac surgery? F1000Res 6: 563.
- Lin Y, Chen L, Li W, Fang J (2015) Role of high-mobility group box-1 in myocardial ischemia/reperfusion injury and the effect of ethyl pyruvate. Exp Ther Med 9(4): 1537-15241.
- Kang R, Zhang Q, Zeh HJ, Lotze MT, Tang D (2013) HMGB1 in Cancer: Good, Bad, or Both? Clin Cancer Res 19(15): 4046-4057.
- Gangemi S, Casciaro M, Trapani G, Quartuccio S, Navarra M, et al. (2015) Association between HMGB1 and COPD: A Systematic Review. Mediators Inflamm 2015: 164913.
- Kohno T, Anzai T, Naito K, Miyasho T, Okamoto M, et al. (2009) Role of high-mobility group box 1 protein in post-infarction healing process and left ventricular remodelling. Cardiovasc Res 81(3): 565-573.
- Andrassy M, Volz HC, Igwe JC, Funke B, Eichberger SN, et al. (2008) High-mobility group box-1 in ischemia-reperfusion injury of the heart. Circulation 117(25): 3216-3226.
- Kitahara T, Takeishi Y, Harada M, Niizeki T, Suzuki S, et al. (2008) High-mobility group box 1 restores cardiac function after myocardial infarction in transgenic mice. Cardiovasc Res 80(1): 40-46.
- Limana F, Esposito G, Arcangelo D, Di Carlo A, Romani S, et al. (2011) HMGB1 attenuates cardiac remodelling in the failing Heart via enhanced cardiac regeneration and miR-206-mediated inhibition of TIMP-3. PLoS ONE 6(6): e19845.






























