Perioperative Transesophageal Echocardiography in Myectomy Procedure -4 Cases Report
Marcello Fonseca Salgado-Filho1,2*, Adrielle Aprigio de Queiroz2, Cassia Franco Matheus2, Julianne Moreira Belo Crolman2, Rafael Teixeira dos Santos2 and Maia Nogueira Crown Guimaraes3
1Intraoperative Transesophageal Echocardiography coordinator, Brazilian Society of Anesthesiology, Brazil
2Santa Casa de Misericordia deJuiz de Fora, Brazil
3Hospital Sirio Libanes, Brazil
Submission: August 08, 2017; Published: August 29, 2017
*Corresponding author: Marcello Fonseca Salgado Filho, Intraoperative TransesophagealEchocardiography Coordenator, Brazilian Society of Anesthesiology, Brazil, Email: mfonsecasalgado@hotmail.com
How to cite this article: Marcello F S F, Adrielle A Q, Cássia F M, Julianne M B C, Rafael T S, Maia N C G. Perioperative Transesophageal Echocardiography in Myectomy Procedure -4 Cases Report. J Cardiol & Cardiovasc Ther 2017; 7(3): 555711. DOI: 10.19080/JOCCT.2017.06.555711
Abstract
Background: Asymmetric septal hypertrophy is the most prevalent form of hypertrophic cardiomyopathy. The aim of this series of cases is to demonstrate the measures performed by perioperative transesophageal echocardiography (TEE) during myectomy procedures.
Methods: A retrospective analysis of the echocardiography exams from patients who underwent myectomy procedure from January 2015 to January 2017 was developed. Were excluded patients who underwent emergency surgeries, combined surgery and patients who had already septal coronary artery alcoholization.
Results: A total of 4 patients were evaluated in the period from 2015 to 2017. They were all women, ASA 3 with a mean age of 61±8,7 years old. The left ventricle outflow tract (LVOT) peak gradient pre-CPB was 114,4±50,6mmHg and post-CPB was 42,4 46,3mmHg. The LVOT mean gradient pre-CPB was 52,4±19,9mmHg and post-CPB was 21,4±25,6mmHg. The mitral coaptation-septal distance (C-sept) pre-CPB was 1,4±0.4cm and post-CPB was 2,2±0,2cm. The vena contracta pre-CPB was 0,5±0,2cm and post-CPB was 0,4 ±0,3cm. The ejection fraction pre-CPB was 66,5±1,3% and post-CPB was 53±2,7%. The CBP clamp time was 50,3±30,7min and the CPB time 82±42,6min. One patient died in the ICU due a low output syndrome after CPB.
Conclusion: The perioperative TEE assisted in the decision making regarding the anatomical structures to be resected, evaluated the severity of mitral regurgitation before and after resection and guided the surgical team regarding the LVOT peak gradient and C-sept distance
Background
Asymmetric septal hypertrophy (ASH) is the most prevalent form of hypertrophic cardiomyopathy [1]. In ASH, the systolic anterior movement (SAM) of the mitral valve develop a dynamic obstruction in the left ventricular outflow tract (LVOT) that can cause low cardiac output and pulmonary edema [2]. Among the forms of treatment, myectomy surgery stands out [1-3]. Patients with labile obstruction, peak LVOT pressure gradients ≥50mm Hg during exercise, resting gradients >30mm Hg and patients with NYHA class II through IV symptoms refractory to medical therapy are the ones that will benefit from the surgical procedure [1]. The aim of this series of cases is to demonstrate the measures performed by perioperative transesophageal echocardiography during myectomy procedures.
Methods
After approval by the Ethics Committee of Santa Casa de Misericordia of Juiz de Fora, retrospective analysis were developed in the echocardiography exams from patients underwent myectomy procedure from January 2015 to January 2017. Were included in these study patients between 18 to 70 years, ASA 3 and 4. The exclusion criteria were emergency surgeries, combined surgery, and patients who had already septal coronary artery alcoholization. All patients were monitored with pulse oximetry, 5-lead cardioscopy, invasive blood pressure, central venous pressure and transesophageal echocardiography (TEE). They were submitted to balanced general anesthesia with ethomidate [0.3mg/kg), fentanyl [5mcg/kg), rocuronium [0.6mg/kg) and sevoflurane 2%. After intubation, the TEE probe was introduced and perioperative TEE exams were performed following the intraoperative TEE SCA/ASE guideline [4]. Parametric data are expressed as mean ± standard deviation and categorical data in absolute numbers and percentages.
Results
A total of 4 patients were evaluated in the period from 2015 to 2017. Preoperative demographic data and preoperative echocardiographic data are shown in Table 1.

ASA: American Society of Anesthesiologists physical status; SAH: Systemic Arterial Hypertension
Perioperative echocardiographic data are shown in Table 2
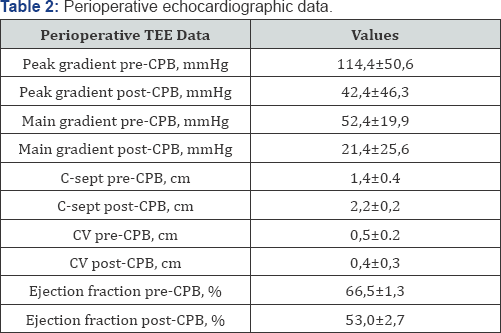
CPB: Cardio Pulmonary By-Pass; C-sep: distance between de mitral valve tip and the septal hipertrophy, CV: Contract Vein
The Figure 1 shows the LVOT obstructed by the anterior mitral leaflet [AMVL).

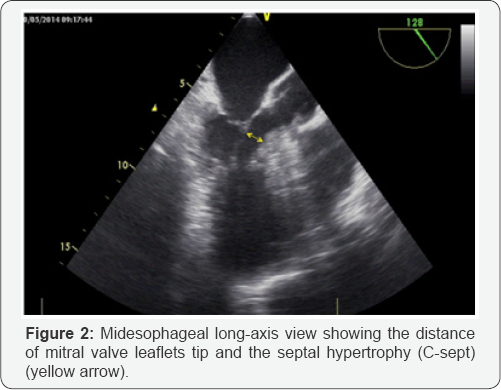
The distance of mitral valve leaflets tip and the septal hypertrophy (C-sept) can be observed in the Figure 2.
In the Figure 3A & 3B can be observed the SAM leading to an important mitral regurgitation and after the cardiopulmonary bypass (CPB), the mitral regurgitation is mild.
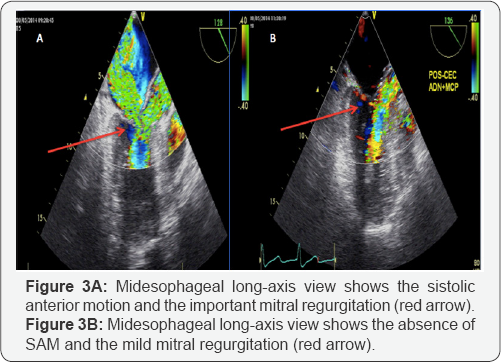
Figure 4 shows the variation of the LVOT peak gradient between the pre-CPB period and the post-CPB period and the assessment of the distance between de mitral valve tip and the septal hypertrophy (C-sept) between the pre-CPB periods with the post- CEC.
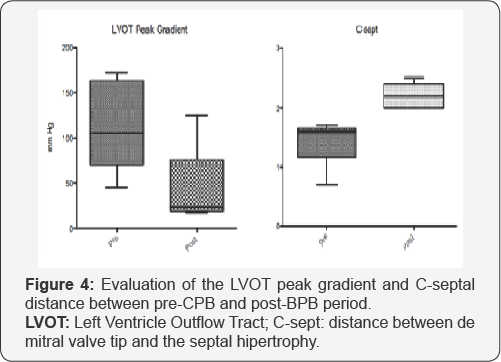
Table 3 shows the clinical outcome of the patients, the time of intubation in the ICU, length of ICU stay and hospital mortality.
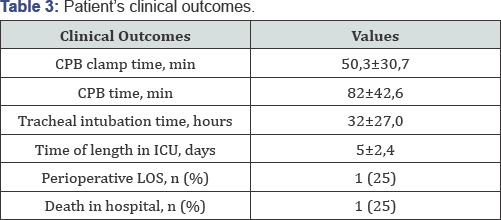
CPB: Cardiopulmonay By-Pass; ICU: Intensive Care Unit; LOS: Low Output Syndrome
Discussion
In these 4 cases of septal myectomy procedure, the perioperative TEE assisted the decision making regarding the anatomical structures to be resected. TEE also showed possible complications of the procedure like ventricular septal perforation, aortic regurgitation, mitral regurgitation and persistent LVOT obstruction [1-3]. We concluded in our study, that myectomy procedure increases the C-sept distance and decreases the SAM, leading a lower LVOT peak gradient and lower mitral regurgitation [1-3].
References
- Hensley N, Dietrich J, Nyhan D, Mitter N, Yee MS, et al. (2015) Hypertrophic cardiomyopathy: A review. Anest analg 120(3): 554-569.
- Hymel BJ, Townsley MM (2014) Echocardiographic assessment of systolic anterior motion of the mitral valve. Anest analg 118(6): 11971101.
- Coyne JT, Alfirevic A (2013) Reorientation of an obstructive, hypermobile papillary muscle: Intraoperative echocardiographic assessment. Anest Analg 116(5): 989-992.
- Hahn RT, Abraham T, Adams MS, Bruce CJ, Glas KE, et al. (2013) Guidelines for performing a comprehensive transesophageal echocardiographic examination: Recommendations from the american society of echocardiography and the society of cardiovascular anesthesiologists. J Am Soc Echocardiogr 26(9): 921-964.






























