A Case of Entrapped Rotablator Burr and Possible Mechanism of Entrapment: Insight from Intravascular Ultrasound Findings
Shuntaro Ikeda*, kiyotaka Ohshima, Haruhiko Higashi, Jun Aono Kouki Watanabe, Akiyoshi Ogimoto and Mareomi Hamada
Department of Cardiology, Uwajima City Hospital, Japan
Submission: August 10, 2017; Published: August 24, 2017
*Corresponding author: Shuntaro Ikeda, Goten-machi, Uwajima, Ehime 798-8510, Japan, Tel: 0898-25-1111; Fax: 0895-25-5334; Email: ikeda.shuntaro@gmail.com
How to cite this article: Shuntaro I, kiyotaka O, Haruhiko H, Jun A K W, Akiyoshi O, et al. A Case of Entrapped Rotablator Burr and Possible Mechanism of Entrapment: Insight from Intravascular Ultrasound Findings. J Cardiol & Cardiovasc Ther 2017; 7(1): 555705.DOI:10.19080/JOCCT.2017.06.555705
Abstrac
The effectiveness of rotational atherectomy has been established for hard, calcified lesions rotablator burr entrapment is a rare complication of rotational atherectomy, estimated to occur in 0.4% of cases. A 54-year-old woman was admitted to our hospital to undergo percutaneous coronary intervention for an atherosclerotic lesion of left anterior descending coronary artery. Coronary angiography revealed diffuse narrowing with severe calcification in her left anterior descending coronary artery, which seemed to be refractory to conventional devices. We decided to perform lesion modification by rotablator. During rotablation, the burr suddenly stalled and became trapped at the distal portion of the left anterior descending coronary artery just beyond the severely calcified lesion. We successfully retrieved the entrapped burr by balloon inflation after the insertion of a second guide wire. IVUS demonstrated that striking characteristic lesion morphology is short, "napkin ring”-like severe calcification within a long, eccentric, calcified lesion. Our IVUS findings suggest that firm, "napkin-ring” calcification refractory to rotablation is a high-risk lesion characteristic for burr entrapment. Interventional cardiologists should pay much attention to the existence of angiographically hidden, "napkin-ring” calcification.
Keywords: Rotational atherectomy; Complication; IVUS
Introduction
The effectiveness of rotational atherectomy as the primary strategy for complex, severe calcified lesions has been established for facilitating device delivery, optimal stent expansion, and good apposition [1-6]. However, the rotablator carries the potential for life-threatening complications. Flow complications, including slow flow/no reflow, coronary perforation, and dissection, are well known. However, rotablator burr entrapment [7-15] is a rare complication of rotational atherectomy, estimated to occur in 0.4% of cases [3]. Herein, we report a case of this rare complication treated with successful retrieval of the entrapped rotablator by balloon inflation after the insertion of a second guide wire. Furthermore, we discuss the mechanism responsible for the burr entrapment based on lesion characteristics obtained from intravascular ultrasound (IVUS) findings.
Case Series
A 54-year-old woman was admitted to our hospital to undergo percutaneous coronary intervention for an atherosclerotic lesion of left anterior descending coronary artery. Her coronary risk factors included hypertension. Two weeks prior to this admission, she experienced unstable angina and underwent medical treatment at another hospital. Diagnostic coronary angiography revealed diffuse narrowing with severe calcification in her left anterior descending coronary artery (LAD), which seemed to be refractory to conventional devices.
Pre-intervention coronary angiography revealed no significant stenosis in the right coronary artery. In contrast, a long calcified lesion was detected in the proximal LAD (Figure 1). An 8- Fr Judkins curve left-type guiding catheter with side holes was inserted from the right femoral artery to the left coronary artery. After intravenous administration of 10,000 units of heparin, a 0.014-inch guide wire (BMW Universal; Abbott Vascular) was crossed. Prior to coronary intervention, we attempted to perform IVUS (Galaxy; Boston Scientific). However, the IVUS catheter could not cross the target lesion, which was thought to be heavily calcified; therefore, we decided to perform lesion modification by rotablator (Boston Scientific). The floppy guide wire was then replaced by a rotawire floppy wire (Boston Scientific) through the narrowing with the aid of a micro-catheter (Finecross; Terumo).

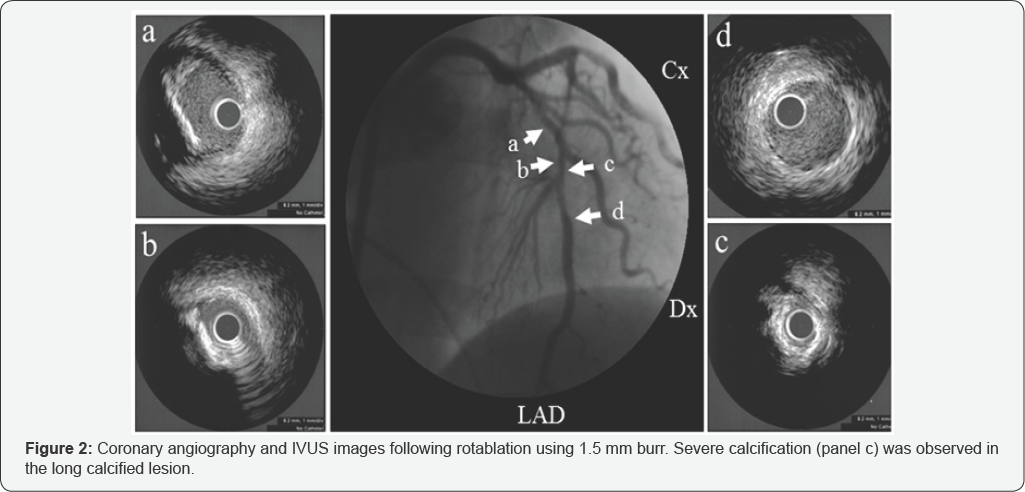
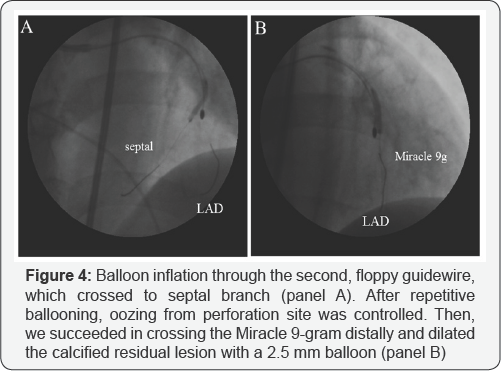
At first, the rotawire crossed the lesion, and we tried to ablate the narrowing using a 1.5mm burr on the rotablator at 180,000rpm. The starting site was 5 mm proximal to the lesion. The method of burr advancement was a pecking motion, and ablation time per session was <15seconds. After ablation with a 1.5mm burr, we examined the state of the coronary artery with IVUS. IVUS findings revealed that the lesion was long, tortuous, and heavily calcified with eccentric calcification (Figure 2], which was very severe at the minimum lumen site (Figure 2: panel c]. IVUS revealed a normal appearance of the distal position adjacent to the severely eccentric calcified lesion (Figure 2: panel d). We attempted to modify the lesion with a large rotablator burr. We began ablation with a 1.75mm burr at 180,000 rpm. After a few pecking motions, the burr suddenly stalled and became trapped at the distal portion of the LAD just beyond the severely calcified eccentric lesion. We advanced the guiding catheter deeply into the left coronary artery and attempted retrieval by pulling the burr with manual traction. However, we could not retrieve the entrapped burr. Although there was still thrombolysis in myocardial infarction grade 2 ante grade coronary flow from the entrapped site, coronary perforation was also noted from distal site of burr entrapment (Figure 3). She complained of chest pain, and electrocardiography demonstrated ST elevation of left precordial leads. Additionally, her systolic blood pressure suddenly decreased to 50 mmHg, so we immediately inserted an intra-aortic balloon pump and temporary pacing catheter. In order to control coronary perforation, we tried to cross a floppy wire to the LAD; however, the floppy wire did not cross. We select the proximal septal branch, and inflated a 2.5mm balloon (Figure 4: panel a). We successfully controlled the oozing from the perforated site with repetitive balloon inflation. The patient's hemodynamic status improved. We inserted a second, stiffer guide wire (Miracle 9- gram: ASAHI INTECC) to the distal LAD in order to dilate the proximal site of burr entrapment. We succeeded in crossing the Miracle 9-gram distally and dilated the calcified residual lesion with a 1.5mm balloon. Then, we changed to a 2.5mm balloon (Figure 4: panel b). Finally, we retrieved the entrapped burr from the LAD. We performed final angiography and IVUS to determine the LAD’s condition (Figure 5). At the left main trunk, coronary dissection was observed (Figure 5a]. At the severely calcified site, ablation remained sub-optimal (Figure 5d). At the site of burr entrapment, the coronary artery was ablated deeply, as with an aneurysm (Figure 5e & 5f].

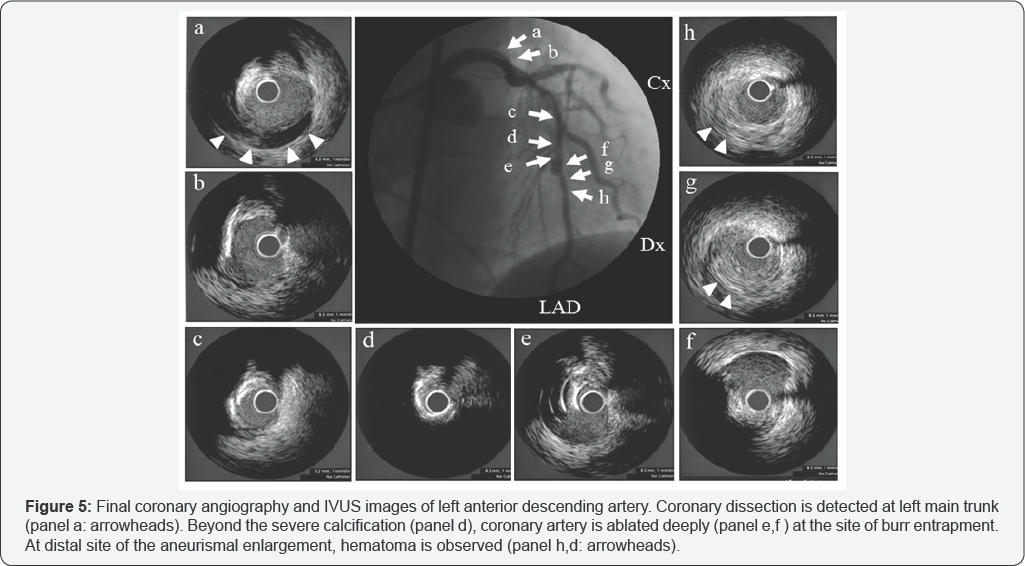
Further percutaneous intervention was deemed inappropriate because of the dissection of the proximal left coronary artery and the perforation of the mid-LAD. Therefore, the patient was referred for emergent coronary artery bypass grafting. The operation was performed uneventfully. She was discharged from our hospital at post-operative day 16.
Discussion
Percutaneous coronary intervention by rotablator is performed in calcified lesions to facilitate ballooning and stent deployment [1-5]. Burr entrapment may occur as a rare, life- threatening phenomenon. In the present case, we tried to perform lesion modification for the calcified lesion by rotablation, resulting in device entrapment. IVUS demonstrated a long, eccentric, calcified lesion with a short, "napkin-ring”-like, severe calcification at the minimal lumen diameter site.
There are some reports regarding entrapment mechanisms [8-15]. Kaneda et al. [8] reported that the "Kokeshi Phenomenon” can contribute to burr entrapment. They noted that the small burr tip can easily pass through the calcified lesion without removing a significant amount of calcification. This phenomenon may occur because the high-speed rotation of the burr can cause transient enlargement of the stenos is by friction heat, resulting in burr entrapment at the distal site of the proximal narrowing. In addition, because the burr was only diamond-coated at the front half of the surface, calcification just proximal to the burr could not be ablated, resulting in burr entrapment. Another mechanism may be the high resistance between a relatively large rotablator burr and the diffuse severe narrowing with heavy calcification, resulting in the burr becoming embedded along the way of the long calcified narrowing. In our case, when the burr was entrapped, coronary angiography demonstrated that antegrade coronary flow was preserved, indicating the presence of space between the burr and the coronary lumen. In view of angiographic findings, the entrapment in our case may have resulted from the "Kokeshi phenomenon".
Many reports have shown that heavily calcified lesions on angiography represent high risk for entrapment. However, at present, there is no identifying characteristic for IVUS to indicate a lesion at high-risk for entrapment. As shown in Figure 3a striking characteristic lesion morphology as evidenced by our observation is short, "napkin ring"-like severe calcification within a long, eccentric, calcified lesion. In addition, after rotablation by a 1.75mm burr, the thickness of calcification or the luminal diameter at the severe calcification remained unmodified as compared with that on pre-intervention IVUS. Our IVUS findings suggest that firm, "napkin-ring” calcification refractory to rotablation is a high-risk lesion characteristic for burr entrapment. Interventional cardiologists should pay attention to the existence of angiographically hidden, "napkin-ring” calcification.
As a bail-out method, the most popular solution is manual traction with deep engagement of the guiding catheter. However, trauma to the proximal part of the coronary artery may limit this maneuver. Second, balloon dilatation of the proximal part of the entrapped rotablator burr may be an effective, non-surgical method [9,11,13]. If there is coronary flow from the entrapped site, second wire insertion and subsequent dilatation may be promising. If a conventional wire could not be passed, operators should attempt to use a stiffer, tapered-tip guide wire (for example, Conquest; Asahi Intec), which may result in avoidance of coronary artery bypass grafting [11]. Prior to second wire or balloon insertion, extracting the drive shaft sheath by cutoff the rotablator system nearby the advancer may be effective for securing space for the second wire and devices [10,12]. This method is expected to be useful when the operator uses a system <8 Fr. In our case, we tried to cross a second guide wire alongside the stuck burr because the floppy guide wire could not be crossed. Therefore, we selected a stiffer wire, resulting in successful crossover to the distal vessel.
Recent reports have shown that retrieval using a "mother and child" catheter is effective. After cutting off the drive shaft and sheath, the "mother and child" catheter is advanced near to the entrapped rotablator burr and pulled together. Kimura et al. reported that they inserted a 5Fr, 120cm straight guiding catheter (Heartrail ST01; Terumo) through the remaining rotablator system after taking off the drive shaft and sheath. They pushed the catheter tip to the lesion and simultaneously pulled the rotablator, resulting in successful retrieval [14]. As a similar method, Cunnings reported successful retrieval using the guideliner (Boston Scientific) [15].
If interventional operators encounter burr entrapment, they should immediately evaluate the necessity of hemodynamic support. If needed, an intra-aortic balloon pump should be quickly inserted and/or temporary pacing should be begun as soon as possible. If the patient's hemodynamic stabilizes, operators should try to cross another guide wire for balloon inflation to retrieve the entrapped burr. If the operator cannot insert a new wire or retrieve the burr using the "mother and child” catheter after extracting the drive sheath, emergent coronary artery bypass grafting must be considered. If the rotablator burr is successfully removed by balloon inflation or the "mother and child catheter," whether percutaneous coronary intervention is continued on the patient's hemodynamic and coronary artery condition. In our case, the trapped rotablator burr itself could likely have been successfully removed percutaneously; however, residual trauma due to coronary intervention required emergent surgical revascularization. Rotational atherectomy appears to be efficacious in the treatment of hard, calcified plaque. However, this incident might have occurred because of quick advancement under insufficient ablation of the calcification. Forced pecking motion may wedge the rotablator into the coronary artery. To avoid entrapment of the rotablator burr, operators should perform careful manipulation, especially for "napkin-ring” calcification submerged by long calcification, as determined by angiography. Interventional cardiologists should be familiar with bail-out methods in the case of life-threatening complications.
Conclusion
We experienced a case with successful retrieval of the entrapped rotablator by balloon inflation after the insertion of a second guide wire. A striking characteristic lesion morphology demonstrated by IVUS is short, "napkin ring”-like severe calcification within a long, eccentric, calcified lesion. Our IVUS findings suggest that firm, "napkin-ring” calcification refractory to rotablation is a high-risk lesion characteristic for burr entrapment. Interventional cardiologists should devote attention to the existence of angiographically hidden, "napkin-ring” calcification.
References
- Warth DC, Leon MB, O Neill W, Zacca N, Polissar NL, et al. (1994) Rotational atherectomy multicenter registry: acute results, complications and 6-month angiographic follow-up in 709 patients. J Am CollCardiol 24(3): 641-648.
- Reisman M, Harms V, Whitlow P, Feldman T, Fortuna R, et al. (1997) Comparison of early and recent results with rotational atherectomy. J Am Coll Cardiol 29(2): 353-357.
- Nobuyoshi M (2000) Experience is the best teacher. Catheter Cardiovasc Interv 49: 85.
- Safian RD, Feldman T, Muller DW, Mason D, Schreiber T, et al. (2001) Coronary angioplasty and Rotablatoratherectomy trial (CARAT): immediate and late results of a prospective multicenter randomized trial. Catheter Cardiovasc Interv 53(2): 213-220.
- Abdel Wahab M, Baev R, Dieker P, Kassner G, Khattab AA, et al. (2013) Long-term clinical outcome of rotational atherectomy followed by drug-eluting stent implantation in complex calcified coronary lesions. Catheter Cardiovasc Interv 81(2): 285-291.
- Brown DL, Buchbinder M (1996) Incidence, predictors and consequences of coronary dissection following high-speed rotational atherectomy. Am J Cardiol 78(12): 1416-1419.
- Gambhir DS, Batra R, Singh S, Kaul UA, Arora R (1999) Burr entrapment resulting in perforation of right coronary artery. Indian Heart J 51(3): 307-309.
- Kaneda H, Saito S, Hosokawa G, Tanaka S, Hiroe Y (2000) Trapped rotablator: Kokeshi phenomenon. Catheter Cardiovasc Interv 49(1): 82-84.
- Grise MA, Yeager MJ, Teirstein PS (2002) A case of an entrapped rotational atherectomy burr. Catheter CardiovascInterv 57(1): 31-33.
- Prasan AM, Patel M, Pitney MR, Jepson NS (2003) Disassembly of rotablator: getting out of a trap. Catheter Cardiovasc Interv 59(4): 463-465.
- Hyogo M, Inoue N, Nakamura R, Tokura T, Matsuo A, et al. (2004) Usefulness of conquest guidewire for retrieval of an entrapped rotablator burr. Catheter Cardiovasc Interv 63: 469-472.
- Sakakura K, Ako J, Momomura SI (2011) Successful removal of an entrapped rotablation burr by extracting drive shaft sheath followed by balloon dilatation. Catheter Cardiovasc Interv 78(4): 567-570.
- De Vroey F, Velavan P, El Jack S, Webster M (2012) How should I treat an entrapped rotational athetectomy burr? Euro Intervention 7(10): 1238-1244.
- Kimura M, Shiraishi J, Kohno Y (2011) Successful retrieval of an entrapped rotablator burr using 5 Fr guiding catheter. Catheter Cardiovasc Interv 78(4): 558-564.
- Cunnington M, Egred M (2012) GuideLiner, a child-in-a-mother catheter for successful retrieval of an entrapped rotablator burr. Catheter Cardiovasc Interv 79(2): 271-273.






























