Stretching the Cardiac Donor Ischemic Time to Beyond Six Hours: What’s the Impact?
Rahul Chandola*
Division of Heart and Lung Transplant, University of Alberta Hospital, Canada
Submission: March 14, 2017; Published: May 26, 2017
*Corresponding author: Rahul Chandola, Division of Heart and Lung Transplant, University of Alberta Hospital, 77 Gerrard street west, Toronto, Ontario, M5G2A1, Canada, Fax: 1 416340-4385; Email: r-chandola@rediffmail.com
How to cite this article: Rahul C. Stretching the Cardiac Donor Ischemic Time to Beyond Six Hours: What’s the Impact?. J Cardiol & Cardiovasc Ther 2017; 5(3): 555664. DOI: 10.19080/JOCCT.2017.05.555664. DOI:10.19080/JOCCT.2017.05.555664
Introduction
An estimated 5.1 million Americans ≥20 years of age have heart failure. By 2030, the prevalence of HF is believed to increase by 25% [1]. The International Society for Heart and Lung Transplantation (ISHLT) registers upto 3,500-4,000 heart transplants worldwide every year. The number of transplants done annually have been quite static over last 20 years despite the growing heart failure population. The shortage of donor hearts has clearly limited the number of heart transplantations [2]. This disparity in organ (heart) demand and the ever increasing shortage of donors has led to a need to expand the donor eligibility criteria. This has led to accepting the donor organs from remote places, with the anticipated prolonged ischemic times. Although donor ischemic times up to 4-5 hours are generally acceptable, the benefits of ischemic times in excess of 240-300 minutes are still arguable [3]. Although many studies have analyzed the impact of long ischemic times on the adult cardiac transplant outcomes in terms of survival and graft function [3-6], these studies have been limited by a relatively small number of patients with longer ischemic time ranges. As a result, the evidence evaluating the impact of prolonged ischemia time, in particular beyond 360 min in the adult population, on the heart transplant outcomes is limited. We sought to determine if prolonged ischemic times beyond 360 minutes has any impact on the short, midterm and long term survival and the postoperative outcomes of heart transplants at University of Alberta.
Methods
Data was collected from the Alberta Provincial Project for Outcome Assessment in Coronary Heart Disease (APPROACH) database. This database is a prospective data collection registry that collects real-time data, from three hospital sites, beginning at the patient’s referral for cardiac catheterization. Data is entered into the APPROACH registry along the patient’s clinical trajectory. The APPROACH database is a mandatory registry for all patients in all hospitals that provide cardiac catheterization, coronary revascularization and cardiac surgery in Alberta, Canada, a province of approximately 3.7 million people.
APPROACH contains demographic data as well as the presence or absence of previous myocardial infarction, congestive heart failure, cerebrovascular disease, peripheral vascular disease, chronic pulmonary disease, elevated creatinine, renal dialysis, hyperlipidemia, hypertension, liver disease, gastrointestinal disease, and malignancy as well as indication for revascularization, extent of CAD, and procedural data including pre, peri and post operative data. Adverse events data are also recorded in the APPROACH database and reviewed through various hospital based morbidity and mortality rounds. As the data in APPROACH is used for both clinical and administrative purposes, software checks have been put into place to ensure that there are limited missing data particularly in the baseline characteristics of the patients in APPROACH. Furthermore, for the purposes of research, we annually employ a data replacement method that has been validated and ensures that the data is over 95% complete. From this database patients are followed longitudinally for the determination of short and long-term outcomes. For the purposes of this study, cardiac catheterization and/or echocardiography were used to measure EF and LVEDP.
Donor acceptance criteria
After establishment of brain death, donor was matched with recipient for ABO blood compatibility and body weight matching. We accept donors within 20% of the recipient weight. Prospective human leukocyte antigen (HLA) matching was not used but the patients with high levels of panel reactive anti-HLA antibodies underwent a prospective cross-match. The donors are accepted preferably under age 60, especially if long ischemic times are expected. The echocardiogram should not reveal any structural heart disease, shunts (except patent foramen ovale), more than mild left ventricular hypertrophy and no significant wall motion abnormalities. Other factors to be considered include hemodynamic stability and not more than small to moderate doses of isotropic support. We prefer to have coronary angiograms for patients >45 years. Coronary angiogram should not reveal anything more than mild coronary artery disease. The donor should not have a history of heart disease, sepsis; known malignancy (some primary brain tumors can be accepted). Serologies should be negative for HIV, hepatitis B (hepatitis B s Ag), hepatitis C, HTLV and syphilis.
Donor exclusion criteria
Donors with ABO incompatibility, body weight and size mismatch and those who do not meet brain death criteria as outlined under policies, are excluded. Other exclusion criteria are HIV positivity, Hepatitis B surface antigenemia, Hepatitis C positivity, evidence of significant infection or sepsis, any structural cardiac deformity, ventricular arrhythmias, more than mild coronary artery disease on angiogram, poor left and/or right ventricular function that does not responds to relevant isotropic, preload and after load manipulations and/or ejection fraction <45% or fractional shortening <25%, echocardiographic evidence of significant valvular abnormality, any acute malignancy (except some primary brain tumors), carbon monoxide poisoning as the cause of death and history of intravenous drug use.
Graft procurement
Goal at this juncture is the effective treatment of potential cardiac arrhythmias, hemodynamic instability, metabolic acidosis and diabetes insipidus. Methyl prednisolone 10- 15mg/kg bolus, Triiodothyronine (T3) 3mcg/hr, Thyroxin (T4) 10mcg/hr, vasopressin 0.5-4 units/hr is started and titrated to keep SVR 850- 1250. Insulin infusion is run at 1 unit/hr and titrated to keep blood glucose levels to 120 -180mg/dl. Donor hearts were harvested from heart-beating, brain-dead individuals. Donor hearts were perfused with 2 litres of Celsior solution at a constant pressure of 60mmHg over a 7-10 min period and were transported immersed in hypothermic celsior solution with normal saline at 4-8C. Biatrial technique described by Lower and Shum way was utilized for transplantation before year 2000 and we changed to bicaval anastomosis technique since then [5,6].
Immunosuppressive regimen
The use of routine induction therapy is the current standard of care for all patients undergoing cardiac transplant at the University of Alberta, with one exception: anti-HCV positive patients do not receive induction. Primary agent used for induction is Rabbit anti-thymocyte globulin (Thymoglobulin®) given at a dose of 0.75mg/kg IV in 250ml of NS via central line to run over 12 hours twice a day for 3 days. The daily dose is continued to maintain absolute lymphocyte count (ALC) ≤0.2x109/L x >2 days. Tacrolimus is started every12 hours once the renal function stabilizes with the dose to be adjusted in response to serum drug levels. Tacrolimus is usually administered orally q12hrs (8 am and 8 pm) beginning post-operatively when bowel sounds are present and renal function is stable (optimally when it is approaching baseline). The initial oral dose is 0.075mg/kg/day administered in two divided doses. At 0-3 months, tacrolimus levels are maintained at 8-12ug/L, at 3-6 months in the range of 6-10ug/L and 6 months onwards at 5-8ug/L.
Since 2000, azathioprine was replaced by mycophenolate mofetil (MMF). Usual dose is 500-1500mg po BID for MMF. Individual dosage adjustments are made according to patient response (GI tolerance) and WBC’s. Methylprednisolone (2mg/ kg) IV is given perioperatively every 12 hours for 3 doses. First dose is started within four hours of patient arrival in CVICU. Prednisone is started at 1mg/kg NG/PO daily, after third dose of methylprednisolone, if bowel sounds are present. The dose is tapered per day to achieve 0.3mg/kg/day by 30 days posttransplant. Tapering doses of prednisone are calculated for each patient individually to achieve the following: 1-month post-op: 0.3mg/kg daily, 2-months post-op: 0.2mg/kg daily, 3-months post-op: 0.1mg/kg daily and further weaning to discontinue prednisone by 6 months.
Recipient exclusion criteria
Exclusion criteria for cardiac transplantation were factors that adversely impact long-term survival (e.g. cancer), increase perioperative morbidity and mortality (e.g. pulmonary hypertension, recent pulmonary embolus, active infection), or affect a patient’s ability to care for himor herself (eg, untreated major psychiatric illness, recent substance abuse). Pretransplant pulmonary hypertension, defined as greater than 6 Woods units, was also considered to be a relative contraindication to transplantation. Many of these comorbidities, however, are being reevaluated, given our favorable experience in transplanting patients once perceived to be high risk (eg. diabetics).
Statistical Analysis
All pre-operative, peri-operative and post-operative categorical variables were compared among the two groups by χ2 test for independence, and continuous variables (age and body mass index) were compared by one-way analysis of variance (ANOVA) with post-hoc Bonferroni correction. Longterm survival after heart transplantation was estimated using Kaplan-Meier actuarial log rank statistics for the two groups. Multi-variate regression using Cox proportional hazards modeling was used to determine independent risk factors for death for all patients analyzed in the cohort.
Results
From January 1992 through January 2012, 475 patients underwent cardiac transplantation at University of Albertahospital. The recipients were separated into the two groups based on length of ischemic time in minutes: group 1, moderate Ischemic Time (MIT), 120-359 minutes (n=382); group 2 prolonged Ischemic Time (PIT), >360 minutes (n=93). All 475 recipients were included in the analysis, including patients who died in the early postoperative period. Ischemic time was defined as the interval from application of donor aortic cross clamp to release of the recipient cross clamp.
Recipient demographics
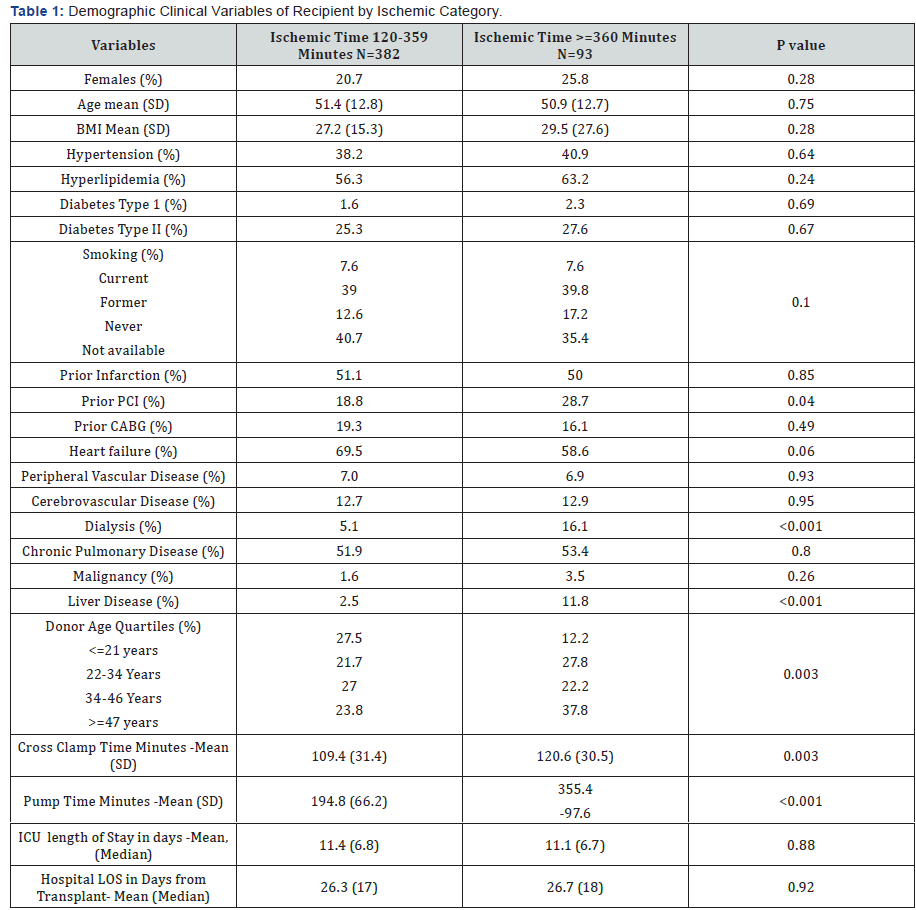
The PIT group, had 69 males (74.2%) 24 females (25.8%), had a mean age at transplantation of 50.9 years, mean BMI at transplantation of 29.5, and a median waiting time of 19.3 days (3.4 to 260.8 days). The MIT group, 303 males (79.3%) and 79 females (20.7%), had a mean age at transplantation of 51.4 yrs (SD -12.8), mean BMI at transplantation of 27.2 (SD -15.3) and a median waiting time for an organ of 10.3 days (0.8 to 57.5days). There were no statistically significant differences between the groups in terms of recipient gender, age, mean BMIs or median waiting time (Table 1). The significant demographic differences were in terms of relatively younger donors in the MIT group (27.5% vs 12.2% donors <21 years and 23.8% vs 37.8% donors >47 years, p value-.003), frequency of prior PCI (with recipients in MIT group having a lower incidence of previous PCI) (18.8% vs 28.7%, p value-0.04), PIT group having higher number of patients who were being treated with dialysis (16.1 vs 5.1 p value-0.001.) and higher number of patients with liver disease (11.8% vs 2.5%, pvalue-<0.001). As would be expected, the PIT group had significantly longer pump times (355.4 vs 194.8 minutes, p value <0.001.) and associated cross clamp times.
Postoperative outcomes
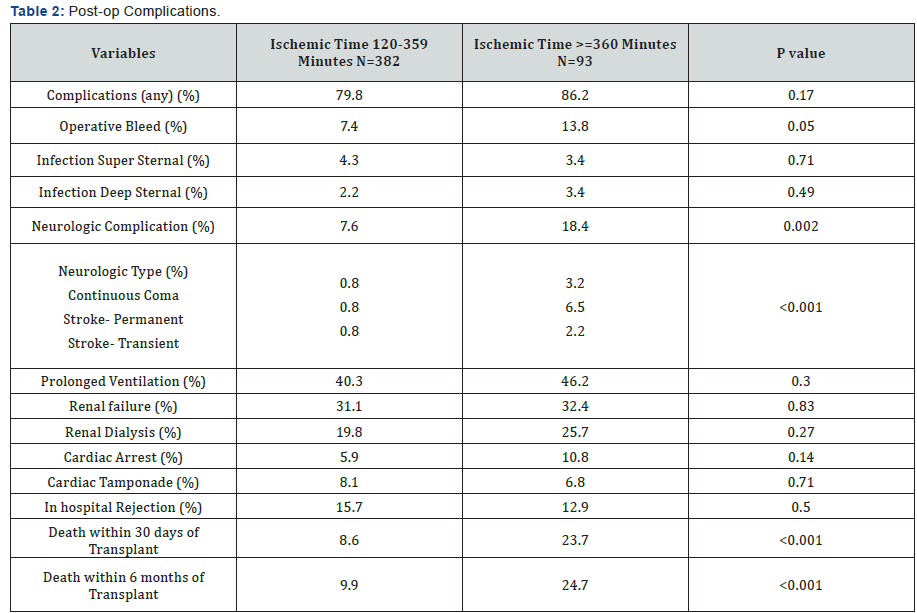
The incidence of postoperative bleeding was significantly higher in PIT group (13.8% vs 7.8%, p value.05) (Table 2). Neurological complications were also quite higher in the PIT group (18.4% vs 7.6%, p value-0.002). Specifically 6.5% of the patients in PIT group had permanent stroke as opposed to 0.8% in the MIT group (p value-<0.001). 2.2% patients in the PIT group had transient stroke as compared to 0.8% in the MIT group (p value <0.001). 3.2% of the patients stayed in continuous coma as opposed to 0.8% of the patients in MIT group (p value <0.001). Incidence of sternal wound infection, duration of ventilation, incidence of postoperative renal dysfunction, incidence of dialysis requirement, cardiac tamponade, cardiac rejection during primary hospitalization were similar between both the groups.
Survival outcomes

Univariate analysis, identified a significant difference between PIT and MIT groups in 30 day (23.7% and 8.6%, p<0.001) and 6 month mortality (24.7% and 9.9% p <0.001). Long term survival between groups was also significantly different (p=0.04) between both groups (Figure 1) Cox proportional hazards models were used to test the independent effect of ischemic time on mortality while controlling for pre, peri and post operative variables that were significantly associated with ischemic time (Table 3).
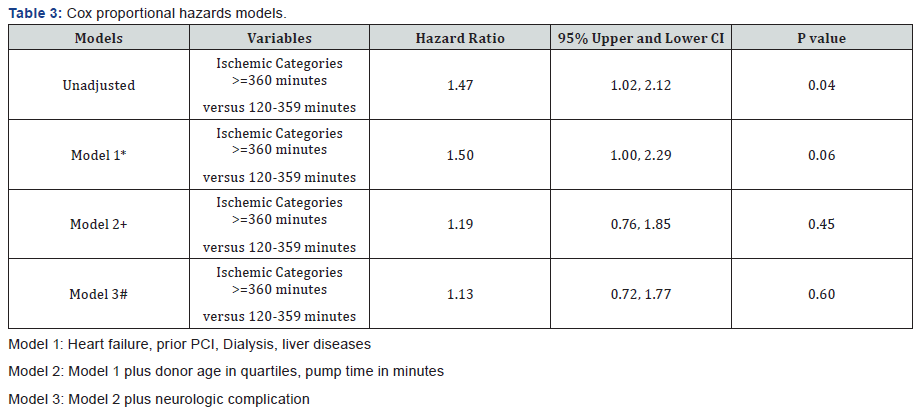
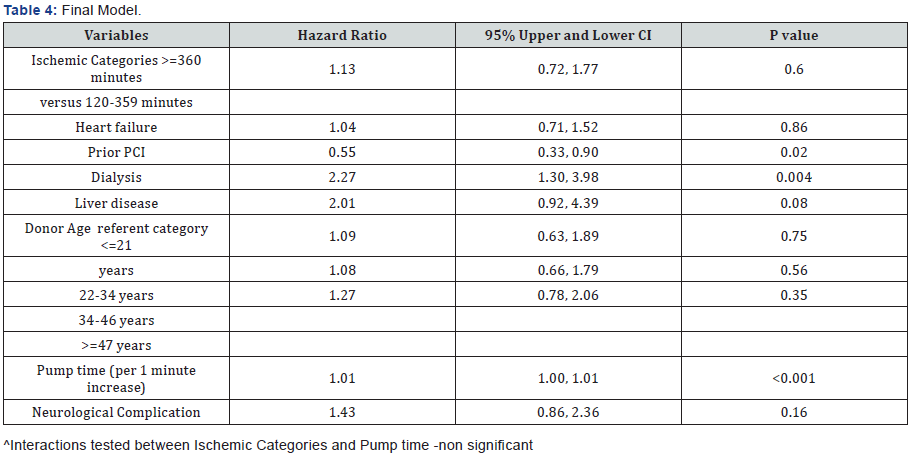
In model 2, following adjustment for donor age and pump time in minutes, the hazard ratio was 1.19 (95% upper and lower CI 0.76 & 1.85.) (p value=0.45). Finally in model 3, we adjusted for neurological complications. The hazard ratio was 1.13 with 95% upper and lower CI of 0.72 and 1.77(p value-0.60) (Table 4).
Discussion
With the ever expanding heart failure population in need of heart transplantation and a shortage of donor organs, cardiac centers often have to accept organs from far off places in an effort to increase the donor pool, leading to prolonged ischemic times. There has been a lot of confusion in regards to effect of donor ischemic times on transplant outcomes. Some of the studies did not find any relationship between ischemic time and survival [7-9]. Some multi-institutional studies, on the other hand, found early mortality after transplantation to be affected by prolonged donor ischemic times [10-12]. There were few other studies that found an inverse relationship between donor ischemic times and survival outcomes [13]. Mullen et al. [14] from our institution compared the groups with donor ischemic times lesser and greater than 4h and did not find any difference in the 30-day, 90-day or actuarial survival between the groups. The use of allograft with ischemia times greater than 4-5h has been reported by some investigators with prolonged ventilation, prolonged ICU stay, prolonged hospital stay, increased graft dysfunction, and higher morbidity and early mortality [15-20]. It was interesting to find, contrary to previous studies, there wasn’t significant difference between the two groups in terms of need for prolonged ventilation (40.3% in MIT vs 46.2% in PIT, p value-0.30), length of ICU stay (11.4 vs 11.1 days, p value-0.88) or duration of hospital stay (26.3 vs 26.7 days, p value-0.92). Interestingly, the length of isotropic support also did not differ significantly between both the groups. The incidence of renal failure and the number of patients requiring dialysis were also not statistically different. 30 day biopsy-proven acute cellular rejection grade 3A or greater was not statistically different in both the group. 15.7% of the patients in MIT group had grade 3A or greater acute cellular rejection compared to 12.9% in the PIT group (p value-0.50). There was a difference in the incidence of postoperative bleeding in both the groups. We found 7.4% incidence of take backs for postoperative bleeding in MIT group as compared to 13.8% in the PIT group (p value-.05). This is possibly a result of increased cardiopulmonary bypass times in PIT group (mean of 355.4 minutes vs 194.8 minutes, p<.001) leading to coagulopathy and platelet dysfunction, accounting for increased take backs in this group.
We also found a significantly high incidence of neurological adverse events in the PIT group (18.4% vs 7.6%, p value-0.02). Specifically 6.5% of the patients in PIT group had permanent stroke as opposed to 0.8% in the MIT group (p value-<0.001). 2.2% patients in the PIT group had transient stroke as compared to 0.8% in the MIT group (p value <0.001). 3.2% of the patients stayed in continuous coma as opposed to 0.8% of the patients in MIT group (p value <0.001). Interestingly, the majority of patients with permanent stroke had ischemic cardiomyopathy as the etiology of heart failure. It is known that patients with ischemic cardiomyopathy have significant atherosclerotic disease and it is possible there was a preexisting burden of cerebrovascular atherosclerotic disease that could have led to increased incidence of strokes in this group as suggested by some earlier studies [21]. We could not find any other specific differences between the two groups of patients with strokes because of the relatively small number of patients with permanent strokes.
On univariate analysis, survival outcomes were quite significantly different between both the groups. In PIT group, the 30 day mortality was 23.7% vs 8.6% for the MIT group. The 6 month mortality was 24.7 for PIT group and 9.9% for the MIT group (p value <0.001). We then used the Cox proportional hazards models to test the independent effect of ischemic time on mortality while controlling for pre, peri and post operative variables that were significantly associated with ischemic time. Both the groups (MIT and PIT) were adjusted for heart failure, prior PCI, preoperative dialysis, preexisting liver disease and donor age in four quartiles. After adjusting the donor ischemic time for these variables, the survival outcomes were not statistically significant (The hazard ratio was 1.19 with 95% upper and lower CI of 0.76 and 1.85, p value-0.45, model 2). When both groups were further adjusted for postoperative neurological outcomes, the hazard ratio decreased further 1.13, p value-0.60.
In this study, higher proportions of patients in PIT group have had coronary intraluminal stenting procedures in the past (28.7 vs 18.8, p value-0.04). This could be because this group had more advanced cardiac disease and ischemic burden. The PIT group also had significantly more number of patients on dialysis (16.1 vs 5.1, p value<0.001) and Pretransplant liver dysfunction (11.8 vs 2.5, p value <0.001) suggesting that they were in advanced stages of cardiomyopathy with multiorgan dysfunction, thereby implying more sicker patients comprising this group. We also had older donors in the PIT group. This was statistically significant (12.2% in PIT vs 27.5% donors in MIT <21 years and 37.8 PIT vs 23.8% MIT donors >47 years, p value-.003). This is probably because even the older donors were accepted for sicker recipients, considering the urgently required donor organ in the PIT group. The duration of cardiopulmonary bypass was longer in patients receiving a graft with long ischemic time (mean of 355.4 minutes for PIT vs 194.8 minutes for MIT group, p<.001). This was because of planned longer reperfusion time in patients with prolonged ischemic times. Although univariate analysis did show significant mortality differences, when we controlled both the groups for these above discussed variables(prior PCI, pretransplant dialysis, pretransplant liver dysfuction, donor age and duration of cardiopulmonary bypass), there were no significant differences in survival outcomes between the two groups [( hazard ratio was 1.19(model 2)]. The decision to accept the donor hearts from far off places with anticipated long ischemic times should be a thoughtful process based on detailed evaluation of recipient and donor specific factors. Variables such as recipient co morbidities specially renal dysfunction and hepatic dysfunction, donor age, donor isotropic use and left ventricular function should be considered in the decision making process.
Prolonged donor ischemic time in itself should not be the deciding factor in not accepting the donor heart, as the postoperative and survival outcomes are not much different in patients with prolonged ischemic times. Our series also indicate that donor hearts with prolonged ischemic times should not be implanted to patients with advanced UNOS status and sicker hearts along with other organ system dysfunction, as indicated by some of the other studies [10-12]. In our series, the difference in survival outcomes in univariate analysis was evident because of more sick patients with multiorgan dysfunction in the PIT group but as both the groups were controlled for other variables associated with prolonged ischemic times, the survival outcomes were no different between the groups.
The largest single-center series to evaluate the effect of prolonged donor ischemic times on transplant outcomes (in adult population) evaluated donor ischemic time up to approximately 5 hours (295.5±37.1 minutes). Prolonged DIT was evaluated for up to 5 hours in a cohort of 17 patients [22]. This study did not analyze whether or not DIT >6 hours adversely affected survival. The evidence evaluating the impact of prolonged ischemia time in particular beyond 360min, on the short and long term outcomes after heart transplantation, is limited and to our knowledge, has not been tested in adult cardiac transplantation. In our study, we have shown that even extending DIT beyond 6hrs did not result in statistically significant differences in survival outcomes. Although univariate analysis did show mortality differences, there were no differences in survival outcomes between the two groups after controlling for other pre, intra and post operative variables that were significantly associated with ischemic time. This study signals that in view of the current donor shortage, donor hearts associated with anticipated prolonged ischemic times should be considered as an option to expand the donor pool.
Limitations
A limitation of this study is that the data were collected in a clinical prospective registry and were observational in nature. However we believe that our study given the size of the sample and the extent of the data collected on all patients adds to the literature in this area. Another limitation is the use of death as the only long-term outcome for hazards modeling, other surrogate end-points including rates of re-admission to hospital, and progression of symptoms would all be valuable outcomes to measure.
Conclusion
In summary, we can conclude that with the current techniques of myocardial preservation, modified reperfusion and the detailed evaluation of recipient and donor factors on a case by case basis, donor hearts with ischemia time greater than 6 hours provide comparable postoperative outcomes and short, intermediate and long term survival results. The prolonged ischemic times, in itself, should not be a contraindication for OHT. Rather a thoughtful donor and recipient matching should be done and consideration should be given to the recipient UNOS status and comorbidities specially renal and hepatic dysfunction, donor age, donor cardiac function and stability.
References
- Heidenreich PA, Trogdon JG, Khavjou OA, Butler J, Dracup K, et al. (2011) Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation 123(8): 933-944.
- Hertz MI (2012) The Registry of the International Society for heart and lung transplantation. Introduction to the 2012 annual reports: New leadership, same vision. J Heart Lung Transplant 31(10): 1045-1095.
- Keck BM, Bennett LE, Fiol BS, Daily OP, Novick RJ, et al. (1998) Worldwide thoracic organ transplantation: a report from the UNOS/ ISHLT International Registry for Thoracic Organ Transplantation. Clin Transpl: 1998: 39-52.
- Russo MJ, Chen JM, Sorabella RA, Martens TP, Garrido M, (2007) The effect of ischemic time on survival after heart transplantation varies by donor age: an analysis of the United Network for Organ Sharing data base. J Thorac Cardiovasc Surg 133(2): 554-559.
- Taylor DO, Edwards LB, Aurora P, Christie JD, Dobbels F, et al. (2008) Registry of the International Society for Heart and Lung Transplantation: twenty-fifth official adult heart transplant report -2008. J Heart Lung Transplant 27(9): 943-956.
- Sievers HH, Leyh R, Jahnke A, Kraatz EG, Herrmannet G, et al. (1994) Bicaval versus atrial anastomoses in cardiac transplantation. J Thorac Cardiovasc Surg 108(4): 780-784.
- Leman NR, Levi DS, Alejos JC, Wetzel GT (2005) Predictors of graft longevity in pediatric heart transplantation. Pediatr Cardiol 26(6): 762-767.
- Mitropoulos FA, Odim J, Marelli D, Karandikar K, Gjertson DA, et al. (2005) Outcome of hearts with cold ischemic time greater than 300 minutes: a case matched study. Eur J Cardiothoracic Surg 28(1): 143- 148.
- Morgan JA, John R, Weinberg AD, Kherani AR, Colletti NJ, et al. (2003) Prolonged donor ischemic time does not adversely affect long-term survival in adult patients undergoing cardiac transplantation. J Thorac Cardiovasc Surg 126(5): 1624-1633.
- Young JB, Naftel DC, Bourge RC, Kirklin JK, Clemson BS (1994) Matching the heart donor and heart transplant recipient. Clues for successful expansion of the donor pool: a multivariable, multi institutional report. The Cardiac Transplant Research Database Group. J Heart Lung Transplant 13(3): 353-365.
- Bourge RC, Naftel DC, Costanzo-Nordin MR, Kirklin JK, Young JB (1993) Pretransplantation risk factors for death after heart transplantation: a multiinstitutional study. The Transplant Cardiologists Research Database Group. J Heart Lung Transplant 12(4): 549-562.
- Boehmer JP (1993) Expanding the donor pool: how far is too far? J Heart Lung Transplant 12(5): 816-818.
- Trulock EP, Edwards LB, Taylor DO, Boucek MM, Keck BM, et al. (2004) The Registry of the International Society for Heart and Lung Transplantation: Twenty-First Official Adult Heart Transplant Report-2004. J Heart Lung Transplant 23(7): 804-815.
- Mullen JC, Bentley MJ, Modry DL, Koshal A (2001) Extended donor ischemic times and recipient outcome after orthotopic cardiac transplantation. Can J Cardiol 17(4): 421-426.
- Wahlers T, Cremer J, Fieguth HG, Dammenhayn L, Albes J, et al. (1991) Donor related variables and 30-day mortality after heart transplantation. J Heart Lung transplant 10: 22-27.
- Del Rizzo DF, Menkis AH, Pfungfelder PW, Novick RJ, McKenzie FN, et al. (1999) The role of donor age and ischemic time on survival following orthotopic heart transplantation. J Heart Lung Transplant 18(4): 310- 319.
- Young JB, Naftel DC, Bourge RC, Kirklin JK, Clemson BS, et al. (1994) Matching the heart donor and heart transplant recipient. Clues for successful expansion of the donor pool a multivariable, multi institutional report. The transplant cardiologists research Database Group. J Heart Lung Transplant 13(3): 353-365.
- Bourge RC, Naftel DC, Costanzo-Nordin MR, Kirklin JK, et al. (1993) Pre transplantation risk factors for death after heart transplantation: a multi-institutional study. The transplant cardiologists research Database Group. J Heart Lung Transplant 12(4): 549-562.
- Fernandez J, Aranda J, Mabbot S, Weston M, Cintron G (2001) Overseas procurement of donor hearts: ischemic time effect on postoperative outcomes. Transplant Proc 33(7-8): 3803-3804.
- Briganti EM, Bergin PJ, Rosenfelt FL, Esmore DS, Rabinov M (1995) Successful long-term outcome with prolonged ischemic time in cardiac allografts. J Heart Lung Transplant 14(5): 845-940.
- Adair JC, Call GK, O’Connell JB, Baringer JR (1992) Cerebrovascular syndromes following cardiac transplantation. Neurology 42(4): 819- 823.
- Morgan JA, John R, Weinberg AD, Kherani AR, Colletti NJ, et al. (2003) Prolonged donor ischemic time does not adversely affect long-term survival in adult patients undergoing cardiac transplantation. J Thorac Cardiovasc Surg 126(5): 1624-1633.






























