Pro-Atherogenic Oxidized Ldl/β2-Glycoprotein I Complexes in Diabetes Mellitus: Antioxidant Effect of Statins
Luis R Lopez1*, Ignacio Garcia-De La Torre2, Eiji Matsuura3 and Paul RJ Ames4
1Corgenix Medical Corporation, USA
2Department of Immunology and Rheumatology, University of Guadalajara, Mexico
3Department of Cell Chemistry, Okayama University, Japan
4 Queen Mary University of London, UK
Submission: February 16, 2017; Published: April 26, 2017
*Corresponding author:Luis R Lopez, Corgenix Medical Corporation, 11575 Main Street, #400, Broomfield, CO 80020, USA, Tel:+1 303 453 8949; Fax: +1 303 457 4519; Email: llopez@corgenxi.com
How to cite this article:Luis R L, Ignacio G-D L T, Eiji M, Paul R A. Pro-Atherogenic Oxidized Ldl/β2-Glycoprotein I Complexes in Diabetes Mellitus: Antioxidant Effect of Statins. J Cardiol & Cardiovasc Ther 2017; 4(5): 555649 . DOI:10.19080/JOCCT.2017.04.555649
Abstract
Premature atherosclerotic cardiovascular disease (CVD) is a well known complication of diabetes mellitus (DM) associated with significant morbidity and mortality. The development of atherosclerosis is largely promoted by oxidative stress and chronic inflammation. Elevated low-density lipoprotein (LDL) is a known atherosclerotic risk factor but LDL must be modified to become atherogenic. Inflammatory-derived reactive oxygen and nitrogen species oxidize LDL (oxLDL) giving rise to lipid peroxides and aldehydes that favor the initiation and progression of atherosclerotic lesions. Beta-2-glycoprotein I (β2GPI) is a lipid binding plasma protein with pleiotropic functions that binds oxLDL via specific oxidative-derived ligands to form pro-atherogenic oxLDL/β2GPI complexes and in this guise exerts a buffering effect upon LDL oxidation. Statin (Rosuvastatin) treatment lowered serum levels of oxLDL/β2GPI complexes in a group of DM patients compared to statin untreated DM patient. The oxLDL/β2GPI decrease was independent from the reduction of cholesterol, LDL and triglycerides but likely dependent on Rosuvastatin reduction of nitrates (NO3-) suggesting that Rosuvastatin may impact on the oxidative metabolism of lipids and/or LDL. In addition, the oxLDL/β2GPI complex may represent a surrogate marker of oxidative inflammation in DM.
Keywords: Diabetes; Oxidative stress; OxLDL/β2GPI complexes; Statins; Atherosclerosis
Introduction
Diabetes mellitus (DM) is the fifth deadliest disease in the United States with an annual economic cost estimated over $100 billion. Cardiovascular disease (CVD) represents the most life threatening consequence of DM accounting for the death of up to 65% of DM patients. Aggressive efforts aimed at treating and controlling the classic CVD risk factors over the last few decades have brought along a marked reduction in CVD morbidity and mortality in the US, though the morbidity and mortality attributable to CVD from DM and obesity continues to show an upward trend [1,2].
The laboratory diagnosis of DM relies on the presence of abnormal fasting glucose and/or an abnormal glucose tolerance test alongside abnormalities of lipid and protein metabolism due to defects in insulin production or activity [3]. All these metabolic abnormalities lead to a pro-atherogenicoxidative inflammatory environment. Recent research has further unraveled the pathogenic mechanisms of CVD in DM mostly due to intrinsic rather than extrinsic factors. Because CVD remains the main cause of death in DM, there is a strong need to identify more specific mechanisms that can be acted upon to develop better CVD prevention and bend down the incidence and mortality curves [4].
Atherosclerosis is a chronic progressive disease (Figure 1) characterized by two low grade inflammatory components, one prevalently systemic that starts early in life affecting the vascular endothelium, monocytes and platelets, and another localized to the arterial wall (plaques) that develops in later adulthood [5,6]. Early identification and intervention is important to prevent disease progression. The complex inflammatory process initiates as oxidative stress (lipoprotein oxidation) and progresses with the participation of immuno-inflammatory mononuclear cells of the innate and adaptive immune system [7,8]. The newly issued American College of Cardiology and American Heart Association (ACC/AHA- 2103) Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults took these concepts into consideration by diverting the focus away from just measuring cholesterol into taking in consideration LDL, statin response and inflammatory biomarkers as more clinically relevant risk factors [9].

Oxidative stress and low grade chronic inflammation (oxidative inflammation) contribute to premature atherosclerotic CVD in DM [10]. Indeed, the abnormal lipid profile of diabetes associates biochemically with lipid peroxidation, a process whereby superoxide radical (O2•- ) released by neutrophils or endothelial cells may attack double bonds of arachidonic acid allowing the formation of oxygen containing cyclic structures termed isoprostanes [11]. Isoprostanes are recognized markers of in vivo oxidative stress and their plasma or urinary concentrations are elevated in DM [12,13]. In the course of oxidative inflammation, endothelial and mononuclear cells also generate additional reactive nitrogen species (RNS) including nitric oxide (NO•) that behaves as a pathogenic mediator and/or as a cytotoxic molecule [14]. However, most of NO• mediated pathogenicity depends on the formation of secondary intermediates such as peroxynitrite anion (ONOO-) and nitrogen dioxide (•NO2) that are more reactive and toxic than NO• [15]. In the presence of superoxide radical (O2•-), NO• gives rise to ONOO-, a strong highly reactive oxidant with very short biological half-life producing nitrated proteins [16].
Reactive oxygen species (ROS) and RNS may exert free radical attack on low-density lipoproteins (LDL) releasing lipid peroxides and highly reactive aldehydes (4-hydroxynonenal) that form specific adducts with lysine inducing the posttranslational modification of lipoproteins, with consequent gain or loss of function. During the same process LDL becomes oxidized (oxLDL) turning into a highly pro-inflammatory and atherogenic [17,18]. Beta2-Glycoprotein I (β2GPI) is a lipidbinding plasma protein involved in thrombosis, fibrinolysis, apoptosis, atherosclerosis and angiogenesis [19]; it binds oxLDL via specific oxidative-derived ligands to form oxLDL/ β2GPI complexes [20]. Elevated plasma levels of oxLDL/ β2GPI complexes were initially described in patients with antiphospholipid syndrome (APS) [21] and systemic lupus erythematosus (SLE) [22], but later found in non-autoimmune chronic inflammatory diseases such as chronic nephropathies, coronary artery disease, myocardial infarction and DM [23,24]. OxLDL and β2GPI have been co-localized in human atherosclerotic lesions by immune-hysto chemical staining implying a pro-atherogenic role [25,26]. In the presence of anti-β2GPI antibodies, macrophages ingest oxLDL/β2GPI complexes at an enhanced rate providing further support for their pro-atherogenic role [27,28]. Current experimental evidence, including in vivo imaging techniques, identified the atherosclerotic lesion as the primary site of oxLDL/β2GPI complex formation [20,29].
In DM, serum levels of oxLDL/β2GPI complexes were particularly elevated in patients with greater intima-media thickness (IMT) [30], but were lower in patients taking statins [24]. These observations indicate that oxLDL/β2GPI complexes may behave as modifiable biomarkers and/or as risk factors for atherothrombotic complications of DM. In addition, the lower oxLDL/β2GPI concentration in DM patients on statins suggested that this class of drugs may prevent or decrease the oxidative modification of LDL possibly by an antioxidant mechanism. Indeed HMG-CoA reductase inhibitors (statins) bear antioxidant properties in addition to their lipid-lowering, anti-thrombotic and anti-inflammatory effects [31,32]. We tested the hypothesis that Rosuvastatin had antioxidant effects in DM by performing an open label interventional trial and observed a significant change in serum oxLDL/β2GPI concentration as the primary endpoint. In this review we discuss the role of oxidative stress in atherogenesis and the antioxidant effect of statins on oxLDL/β2GPI complexes.
Oxidative Inflammation and Atherogenesis in DM
The pathogenesis of atherosclerosis in DM is multi factorial:
- Chronic hyperglycemia from insulin deficiency [33,34].
- Chronic dyslipidemia characterized by decreased high-density lipoprotein (HDL), changes in the HDL subpopulations, raised triglycerides, and unchanged or only slightly elevated low-density lipoprotein (LDL) [35].
- Metabolic syndrome characterized by obesity, dyslipidemia, hypertension and insulin resistance [36]. All three promote increased oxidative stress that initiate and perpetuate vascular damage and atherothrombotic complications [37].
Under physiologic conditions, oxidation should be well counteracted by natural enzymatic and non-enzymatic antioxidant mechanisms. In DM, oxidation overrides antioxidant mechanisms [38,39] and initiates endothelial dysfunction by favoring the expression of a pro-adhesive and pro-thrombotic surface that allow the migration of immunoinflammatory cells into the arterial wall (Figure 1). There, local pro-chemotactic and inflammatory cytokines further recruit and activate immuno-inflammatory cells that propagate lipid accumulation, oxidative inflammation and the development of the typical progressive atherosclerotic lesions (plaques) [40,41]. Moreover, early inflammation increases the expression of cell surface receptors and the intracellular accumulation of oxLDL by local arterial mononuclear cells process mediated by scavenger and Fcγ receptors [28].
Multiple efforts by several groups aimed at enhancing the antioxidant defense in DM and CVD patients. Serum and urine bio makers of systemic oxidative stress correlated with blood glucose levels and responded to anti-diabetic intervention[42,43]. In vivo s tudies i ndicated t hat o xidative s tress f rom hyperglycemia starts well before clinical complications become evident, underscoring the importance of glucose control to minimize long term complications of oxidative inflammation in DM. Metformin treatment lowered urinary excretion of 8-isoPGF2a and 11dhTxB2 in newly diagnosed DM patients suggesting that despite a good metabolic improvement, metformin also behaved as an antioxidant and antithrombotic agent in DM [44]. Some epidemiological studies have demonstrated a weak inverse relationship between stroke risk and ingestion of antioxidant foods. Other clinical trials have shown conflictive results regarding the protective effect of antioxidants against CVD outcomes [45,46]. Several ongoing clinical trials are assessing the effectiveness of statins from an antioxidant perspective; so far these studies have suggested a close relationship between oxidative inflammation and atherogenesis but the usefulness of antioxidant-based therapeutics on CVD remains controversial.
Atherogenic oxLDL and oxLDL Complexes
Oxidation of LDL is a key contributor to the initiation and progression of atherosclerosis [7,47] and is a complex process, in going from “minimally oxidized” to more “extensively oxidized” LDL particles induces the expression of adhesion molecules on endothelial cells and the release of chemotactic cytokines into the circulation [48]. These events allow blood monocytes to adhere to the arterial wall and to migrate into the arterial intima, where they differentiate into macrophages. In turn, these activated macrophages enhance a pro-oxidant environment of the arterial wall, causing intensive oxidative modification of LDL lipoproteins including cholesteryl esters, phospholipids and apolipoprotein B [49]. Because oxLDL becomes unrecognizable by LDL receptors, it is taken up by scavenger receptors, which facilitate a persistent intracellular accumulation of LDL by macrophages [50] transforming them into the characteristic foam cells. As the lesion evolves, these elements contribute to the morphological changes that characterize the vulnerable plaques with an unstable lipidrich necrotic core. Advanced lesions may undergo a necrotic breakdown and plaque rupture that precipitate intra-vascular thrombosis with acute occlusion clinically expressed as unstable angina, myocardial infarction, stroke, and/or sudden cardiac death [51].
A lthough t he o xidation o f L DL o ccurs p rimarily i n t he vascular wall, recent studies have provided evidence for the presence of oxLDL in blood [52]. Indeed numerous studies have established oxLDL as an effective marker for the presence of atherosclerosis, detecting both subclinical disease and more advanced or severe CAD [53,54]. Because oxLDL is highly unstable with a very short half-life (30 seconds) in the systemic circulation [55], it is difficult to measure accurately by common immunoassays. In addition, some lipid binding plasma proteins such as β2GPI interact with circulating oxidized lipoproteins tobuffer their deleterious effects. This may cause reduced assay sensitivity and false-negative results as most of the oxLDL assays use monoclonal antibodies directed against just one or a few of the epitopes present on lipid or protein moieties. This phenomenon has hampered the use of oxLDL in CVD clinical trials and clinical laboratory to assess its predictive role in atherogenesis.
Because immune-staining of human atherosclerotic lesions co-localized β2GPI with oxLDL, the relationship between these molecules was further investigated [25,26]. β2GPI is a 50-kDa single-chain phospholipid-binding plasma protein composed of 326 amino acid residues arranged in 5 homologous repeats or domains. The fifth domain contains a positively charged amino acid patch important in anionic phospholipid and oxLDL binding [56]. Unlike native LDL, β2GPI binds oxLDL via specific oxidative-derived ligands to form stable and pro-atherogenic oxLDL/β2GPI complexes [20,57] in an attempt what to quench in an antioxidant fashion the pro-inflammatory and pro-atherogenic effects of oxLDL. But in doing so, oxLDL/ β2GPI complexes also become immunogenic triggering the production of pro-atherothrombotic auto antibodies and immune complexes.
It is now recognized that the immune system plays a role in blood coagulation. Autoimmune-mediated thrombosis refers to auto antibodies that promote venous and arterial thromboembolic events in patients with systemic lupus erythematosus and antiphospholipid syndrome who develop premature atherothrombotic CVD with significant morbidity and mortality [58,59]. Endogenous pro-atherogenc oxLDL/ β2GPI complexes initially described in autoimmunity [22] have been associated with the development of atherosclerotic CVD in non-autoimmune diseases [23,24]. Serum levels in higher oxLDL/β2GPI quartiles were associated with an geographically determined disease severity and give a 3.5 risk for adverse outcomes in acute coronary syndromes [60,61]. Interestingly, statin treatment reduced oxLDL/β2GPI complexes independently from LDL-lowering effects likely via an antioxidant mechanisms [62,63]. Thus, oxLDL/β2GPI complexes meet current criteria for biomarkers of CVD risk:
- To have a direct mechanistic relevance to atherosclerosis (causal relationship).
- To be measured quantitatively with available technology that is accurate, reproducible and cost effective.
- To permit patient stratification for severity and outcomes.
OxLDL/β2GPI and its immune complexes up-regulate the macrophage expression of scavenger and Fcγ receptors, favoring enhanced oxLDL/β2GPI uptake followed by its rapid accumulation in lysosomes where an immune response (innate and adaptive) may be mounted. Experiments evaluating the intracellular trafficking of β2GPI within macrophages showed that free β2GPI was poorly incorporated in late endosomes and stagnated there, whereas complexed β2GPI (to phosphatidylserine liposomes or oxLDL) was quickly transported to lysosomes; the addition of antibodies to β2GPI further accelerated this process [64]. β2GPI auto reactive CD4+ T cells have been identified in patients with APS that preferentially recognized a cryptic peptide (residues 276- 290) in β2GPI domain V that contains the phospholipid-binding site. Macrophages stimulated with phospholipid-bound β2GPI induced an immune response to peptide 276-290 in a HLA-DRrestricted manner, while β2GPI or phospholipids alone did not [65]. In this respect, β2GPI can be viewed as a component of the innate immunity; but once bound to oxLDL, the complex may shift to the generation and maintenance of an adaptive immune response that play an important role in atherogenic inflammation via the inflammasome/IL-1B system [39].
Effect of Statins on oxLDL/β2GPI Complexes
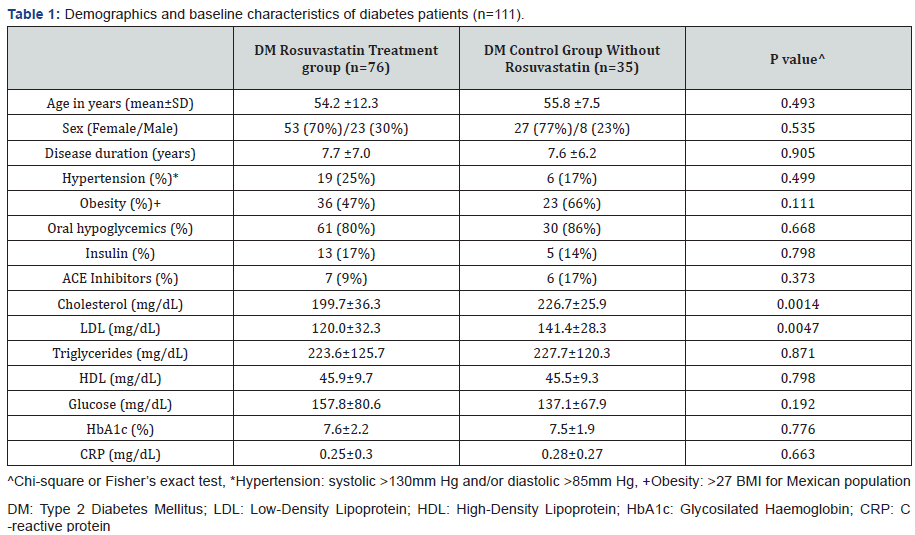
OxLDL/β2GPI complexes are indicative of systemic oxidative inflammation in obese middle age men and DM, and may be used to assess pro-atherogenic pathways because circulating levels of oxLDL independently predict future CVD events [24,66,67]. It was particularly important to determine effective ways to modify oxLDL/β2GPI levels as these complexes have been associated with the severity and adverse outcomes of coronary disease [60,61]. The effect of statins on oxLDL/β2GPI complexes was studied by our group [62] on 111 type 2 DM patients (80 females, 31 males, mean age of 54.7 years). One group of 76 patients received 10mg daily for 6 weeks of oral Rosuvastatin while a control group of 35 patients did not receive Rosuvastatin. Serum samples taken at baseline and after 6 weeks were tested at the end of the study. The baseline clinical and laboratory variables of DM patients taking Rosuvastatin and control groups are shown in Table 1. DM patients in the Rosuvastatin group were stratified according to their lipid profile. In addition to oxLDL/β2GPI complexes, nitrite (NO2-), nitrate (NO3-), asymmetric dymethyl arginine (ADMA) nitrotyrosine (NT) and paraoxonase activity (PON) were measured in all samples.
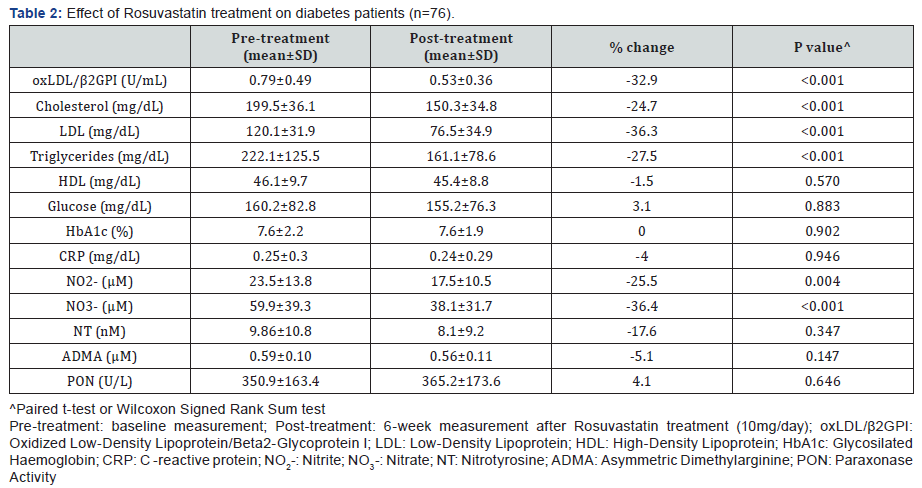
Rosuvastatin treatment caused a significant decrease of oxLDL/β2GPI complexes (32.9%) along with cholesterol (24.7%), LDL (36.3%) and triglycerides (27.5%). Among the nitric oxide metabolites, Rosuvastatin treatment also decreased NO2- (25.5%) and NO3- (36.4%) (Table 2). The observed decrease of oxLDL/β2GPI complexes was more noticeable in patients with dyslipidemia (37.4%) compared to those with normal lipid profile (22.4%). Interestingly, NO2- decreased more in dyslipidemics than in non-dyslipidemic patients (29% vs 18.8%) while NO3- decreased in the same way (42.9% vs 21.8%). The decrease of oxLDL/β2GPI complexes by Rosuvastain t reatment in these DM patients was independent of the lipid lowering effects of the statin. Further, only NO3- was an independent predictor of oxLDL/β2GPI complexes (t=2.0, p=0.04).
Support to an antioxidant effect of statin indirectly assessed by a decrement of oxLDL/β2GPI complexes comes from very few studies.
- A randomized, double blind, placebo controlled pilot study of 37 consecutive SLE patients receiving 40mg daily atorvastatin or placebo for 12 months demonstrated a decrease of oxLDL/β2GPI complexes [63]. In this study, after correction for age and disease duration oxLDL/β2GPI complexes decreased by 27% (p=0.002).
- Blinden et al. [68] studied the effect of statin therapy (Atorvastatin, Simvastatin, Rosuvastatin, Lovastatin, Pravastatin and Fluvastatin at doses between 5-80mg) in 186 coronary artery disease patients undergoing elective cardiac catheterization. There was a significant dosedependent reduction of oxLDL/β2GPI complexes, more noticeable at atorvastatin dose equivalents between 20- 80mg.
- Statin influence of oxLDL/β2GPI levels on CVD patients have been further confirmed by Berger et al. [69] and Gurbel et al. [70]. This effect was independent and inversely associated with inflammation. These finding support the concept of a dose dependent anti-oxidant effect of statins.
Discussion
Our evaluation of the significance of oxLDL/β2GPI complexes in DM demonstrated an independent association with some clinical (obesity and hypertension) and biochemical variables (nitric oxide metabolites) [62]. OxLDL/β2GPI complexes were higher in males than females. This gender difference reflects the notion that in DM oxidative inflammation is enhanced [71] particularly in men [72]. With regards to biochemical variables the only independent predictor of oxLDL/β2GPI was nitrate (NO3-). This RNS may be viewed as an “inflammatory metabolite” of NO• (as opposed to NO2- that may be viewed as the “vascular” metabolite). Thus, NO3- may contribute to LDL oxidation and formation of the oxLDL/β2GPI complex in DM [20].
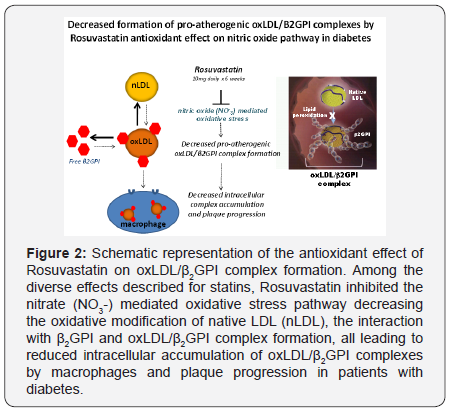
Rosuvastatin administered daily for 6 weeks caused a significant reduction of serum oxLDL/β2GPI complexes (Figure 2). This reduction was accompanied by lower total cholesterol, LDL and tryglicerides, particularly in patients with dislipidemia. However, the reduction of oxLDL/β2GPI was statistically independent of any statin-mediated decrease of total cholesterol, LDL and tryglicerides. It is important to point out that oxLDL/β2GPI levels were higher in DM patients with dyslipidemia, consistent with the concept that patients with elevated lipid levels may be prone to or sustain more intense oxidative damage.
Statins inhibit the enzyme HMG-CoA reductase, preventing the generation of mavelonate and the subsequent biosynthesis of cholesterol. Mevalonate is also a precursor of isoprenoid intermediates and one of these geranylgeranylated proteins (RhoA) is implicated in intracellular signaling [73,74]. Through the inhibition of protein prenylation, such as Ras and Rho, statins activate the MAPK cascade or NF-κB pathways that induce proteins with anti-inflammatory, anti-proliferative and anti-thrombotic effects [75]. In addition, by acting on SREBP-2, statins up-regulate the expression of genes coding for paraoxonase, the enzyme that accounts for most of the antioxidant effect of HDL [76]. Thus, the inhibition of RhoA by statins have a number effects on the vasculature that could be beneficial in hypercoagulable disorders by improving nitric oxide synthase activity, regulation of angiogenesis, reduction of vascular inflammatory and prothrombotic activities and atherosclerotic plaque stabilization [77,78]. By using plasma biomarkers of oxidation such as oxLDL/β2GPI, we can clinically evaluate the effect of treatment on this event.
Because the benefits of statins on the cardiovascular system are beyond those on cholesterol metabolism we speculated t hat Rosuvastatin may exert an antioxidant effect, either by enhancing the activity of PON and of nitric oxide synthase or by interfering with oxidative inflammatory mechanisms that promoted the generation of oxLDL and their consequent interaction with β2GPI [79-82].
Our studies suggest that statins would have the same antioxidant effect on oxLDL/β2GPI complex formation in patients with metabolic syndrome and obesity. Fatty liver disease, particularly non-alcoholic steatohepatitis (NASH), is not only associated with insulin resistance, obesity, metabolic syndrome, liver fibrosis/cirrhosis, but also with atherosclerotic CVD [83]. It has been proposed that dyslipidemia, inflammation, oxidative stress and macrophage activation are early events in NASH, similar to atherosclerosis and perhaps they represent shared aspects of a similar disease process [84]. In this case, stains may have a more prominent therapeutic role as antioxidants are considered first line treatment for NASH.
Conclusion
These studies demonstrate that treatment with Rosuvastatin reduced serum levels of oxLDL/β2GPI in DMpatients. The implications of these findings are twofold: statins independently reduce lipids and NO3- suggesting an antioxidant effect possibly mediated via lipid/nitric oxidative pathways; and that oxLDL/β2GPI complexes may be viewed as serologic biomarkers of oxidative stress.
Acknowledgement
Authors thank Beth L Hurley and Ivana J Muncie for their technical expertise and assistance in testing the samples and organizing the data.
References
- Grundy SM, Benjamin IJ, Burke GL, Chait A, Eckel RH, et al. (1999) Diabetes and cardiovascular disease: a statement for healthcare professionals from the American Heart Association. Circulation 100(10): 1134-1146.
- Grundy SM, Howard B, Smith S, Eckel R, Redberg R, et al. (2002) Prevention Conference VI: Diabetes and cardiovascular disease: executive summary: conference proceeding for healthcare professionals from a special writing group of the American Heart Association. Circulation 105(18): 2231-2239.
- (1985) Diabetes mellitus: Report of a WHO Study Group. World Health Organ Tech Rep Ser 727: 1-113.
- Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, et al. (2011) Heart disease and stroke statistics-2011 Update: A report from the American Heart Association. Circulation 123(4): e18-e209
- Libby P (2002) Inflammation in atherosclerosis. Nature 420(6917): 868-874.
- Hansson GK (2005) Inflammation, atherosclerosis and coronary artery disease. N Engl J Med 352(16): 1685-1695
- Ishigaki Y, Oka Y, Katagiri H (2009) Circulating oxidized LDL: a biomarker and a pathogenic factor. Curr Opin Lipidol 20(5): 363-369.
- Profumo E, Buttari B, Rigano R (2011) Oxidative stress in cardiovascular inflammation: its involvement in immune responses. Int J Inflam 2011: 295705.
- Stone NJ, Robinson J, Lichtenstein AH, Bairey Merz CN, Blum CB, et al. (2014) 2013 ACC/AHA Guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: A report of the American College of Cardiology/American Heart Association task force on practice guidelines. Circulation 129 (Suppl 2): S1-S45.
- Giugliano D, Ceriello A, Paolisso G (1996) Oxidative stress and diabetic vascular complications. Diabetes Care 19(3): 257-267.
- Nourooz-Zadeh J, Cooper MB, Ziegler D, Betteridge DJ (2005) Urinary 8-epi-PGF2alpha and its endogenous beta-oxidation products (2,3-dinor and 2,3-dinor-5,6-dihydro) as biomarkers of total body oxidative stress. Biochem Biophys Res Commun 330(3): 731-736.
- Gopaul NK, Anggård EE, Mallet AI, Betteridge DJ, Wolff SP, et al. (1995) Plasma 8-epi-PGF2 alpha levels are elevated in individuals with noninsulin dependent diabetes mellitus. FEBS Lett 368(2): 225-229.
- Davì G, Ciabattoni G, Consoli A, Mezzetti A, Falco A, et al. (1999) In vivo formation of 8-iso-prostaglandin F2 alpha and platelet activation in diabetes mellitus: effects of improved metabolic control and vitamin E supplementation. Circulation 99(2): 224-229.
- Thomas DD, Ridnour LA, Isenberg JS, Flores-Santana W, Switzer CH, et al. (2008) The chemical biology of nitric oxide: Implications in cellular signalling. Free Radic Biol Med 45(1): 18-31.
- Pacher P, Beckman JS, Liaudet L (2007) Nitric oxide and peroxynitrite in health and disease. Physiol Rev 87(1): 315-324.
- Peluffo G, Radi R (2007) Biochemistry of protein tyrosine nitration in cardiovascular pathology. Cardiovasc Res 75(2): 291-302.
- Malle E, Marsche G, Arnhold J, Davies MJ (2006) Modification of lowdensity lipoprotein by myeloperoxidase-derived oxidants and reagent hypochlorous acid. Biochim Biophys Acta 1761(4): 392-415.
- Leeuwenburgh C, Hardy MM, Hazen SL, Wagner P, Oh-ishi S, et al. (1997) Reactive nitrogen intermediates promote low-density lipoprotein oxidation in human atherosclerotic intima. J Biol Chem 272(3): 1433-1436.
- Matsuura E, Shen L, Matsunami Y, Quan N, Makarova M, et al. (2010) Pathophysiology of beta2-glycoprotein I in antiphospholipid syndrome. Lupus 19(4): 379-384.
- Kobayashi K, Kishi M, Atsumi T, Bertolaccini ML, Makino H, et al. (2003) Circulating oxidized LDL forms complexes with β2-glycoprotein I: implications as an atherogenic autoantigen. J Lipid Res 44(4): 716-726.
- Ames PR, Antinolfi I, Ciampa A, Batuca J, Scenna G, et al. (2008) Primary antiphospholipid syndrome: a low-grade auto-inflammatory disease? Rheumatology 47(12): 1832-1837.
- Lopez D, Garcia-Valladares I, Palafox-Sanchez CA, De La Torre IG, Kobayashi K, et al. (2004) Oxidized low-density lipoprotein/ β2-glycoprotein I complexes and autoantibodies to oxLig-1/β2- glycoprotein I in patients with systemic lupus erythematosus and antiphospholipid syndrome. Am J Clin Pathol 121(3): 426-436.
- Kasahara J, Kobayashi K, Maeshima Y, Yamasaki Y, Yasuda T, et al. (2004) Clinical significance of serum oxidized low-density lipoprotein/ β2-glycoprotein I complexes in patients with chronic renal diseases. Nephron Clin Pract 98(1): 15-24.
- Lopez LR, Hurley BL, Simpson DF, Matsuura E (2005) Oxidized lowdensity lipoprotein/β2-glycoprotein I complexes in type 2 diabetes mellitus. Ann NY Acad Sci 1051: 97-103.
- Ylä-Herttuala S, Palinski W, Rosenfeld ME, Parthasarathy S, Carew TE, et al. (1989) Evidence for the presence of oxidatively modified lowdensity lipoprotein in atherosclerotic lesion of rabbit and man. J Clin Invest 84(4): 1086-1095.
- George J, Harats D, Gilburd B, Afek A, Levy Y, et al. (1999) Immunolocalization of β2-glycoprotein I (apolipoprotein H) to human atherosclerotic plaques: potential implications for lesion progression. Circulation 99(17): 2227-2230.
- Liu Q, Kobayashi K, Furukawa J, Inagaki J, Sakairi N, et al. (2002) ω-Carboxyl variants of 7-ketocholesteryl esters are ligands for β2- glycoprotein I and mediate antibody-dependent uptake of oxidized LDL by macrophages. J Lipid Res 43(9): 1486-1495.
- Matsuura E, Kobayashi K, Inoue K, Lopez LR, Shoenfeld Y (2005) Oxidized LDL/β2-glycoprotein I complexes: new aspects in atherosclerosis. Lupus 14(9): 736-741.
- Sasaki T, Takenaka F, Kita H, Hirano L, Shen K, et al. (2014) Significant accumulation of oxLDL/Beta2-glycoprotein complexes in arterial lesions of WHHL rabbits: PET/CT imaging using an autoantibody’s SCFV variant. Atherosclerosis 235(2): e68.
- Tabuchi M, Inoue K, Usui-Kataoka H, Kobayashi K, Teramoto M, et al. (2007) The association of C-reactive protein with an oxidative metabolite of LDL and its implication in atherosclerosis. J Lipid Res 48(4): 768-781.
- Undas A, Brummel-Ziedins KE, Mann KG (2005) Statins and blood coagulation. Arterioscler Thromb Vasc Biol 25(2): 287-294.
- Shishehbor MH, Brennan ML, Aviles RJ, Fu X, Penn MS, et al. (2003) Statins promote potent systemic antioxidant effects through specific inflammatory pathways. Circulation 108(4): 426-431.
- Baynes JW, Thorpe SR (1999) Role of oxidation stress in diabetic complications in diabetes. A new perspective on an old paradigm. Diabetes 48(1): 1-9.
- Sampson MJ, Gopaul N, Davies IR, Hughes DA, Carrier MJ (2002) Plasma F2 isoprostanes: direct evidence of increased free radical damage during acute hyperglycemia in type 2 diabetes. Diabetes Care 25(3): 537-541.
- De Cristofaro R, Rocca B, Vitacolonna E, Falco A, Marchesani P, et al. (2003) Lipid and protein oxidation contribute to a prothrombotic sate in patients with type 2 diabetes mellitus. J Thromb Haemost 1(2): 250- 256.
- Holvoet P, Lee DH, Steffes M, Gross M, Jacobs DR (2008) Association between circulating oxidized low-density lipoprotein and incidence of the metabolic syndrome. JAMA 299(19): 2287-2293.
- Grant PJ (2007) Diabetes as a prothrombotic condition. J Intern Med 262(2): 157-172.
- Refsgaard HH, Tsai L, Stadtman ER (2000) Modifications of proteins by polyunsaturated fatty acid peroxidation products. Proc Natl Acad Sci 97(2): 611-616.
- Pitocco D, Zaccardi F, Di Stasio E, Romitelli F, Santini SA, et al. (2010) Oxidative stress, nitric oxide and diabetes. Rev Diabet Stud 7(1): 15-25.
- Itable H, Obama T, Kato R (2011) The dynamics of oxidized LDL during atherogenesis. J Lipids 2011: 418313.
- Matsuura E, Lopez LR, Shoenfeld Y, Ames PRJ (2012) B2-glycoprotein I and oxidative inflammation in early atherogenesis: a progression from innate to adaptive immunity. Autoimmun Rev 12(2): 241-249.
- Nathan DM, Cleary PA, Backlund JY, Genuth SM, Lachin JM, et al. (2005) Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 353(25): 2643-2653.
- Paniagua JA, López-Miranda J, Pérez-Martínez P, Marín C, Vida JM, et al. (2005) Oxidized-LDL levels are changed during short-term serum glucose variations and lowered with statin treatment in early Type 2 diabetes: a study of endothelial function and microalbuminuria. Diabet Med 22(12): 1647-1656.
- Formoso G, De Filippis EA, Michetti N, Di Fulvio P, Pandolfi A, et al. (2008) Decreased in vivo oxidative stress and decreased platelet activation following metformin treatment in newly diagnosed type 2 diabetic patients. Diabetes Metab Res Rev 24(3): 231-237.
- Johansen JS, Harris AK, Rychly DJ, Ergul A (2005) Oxidative stress and the use of antioxidant in diabetes: linking basic science to clinical practice. Cardiovasc Diabetol 4: 5.
- Wilcox BJ, Curb JD, Rodriguez BL (2008) Antioxidants in cardiovascular health and disease: key lessons from epidemiologic studies. Am J Cardiol 101(10A): 75D-86D.
- Witztum JL, Steinberg D (1991) Role of oxidized low density lipoprotein in atherogenesis. J Clin Invest 88(6): 1785-1792.
- Itabe H, Mori M, Fujimoto Y, Higashi Y, Takano T (2003) Minimally modified LDL is an oxidized LDL enriched with oxidized phophatidylcholines. J Biochem 134(3): 459-465.
- Frostegård J, Ulfgren AK, Nyberg P, Hedin U, Swedenborg J, et al. (1999) Cytokine expression in advanced human atherosclerotic plaques: dominance of pro-inflammatory (Th1) and macrophage-stimulating cytokines. Atherosclerosis 145(1): 33-43.
- Packer M, Medina N, Yushak M (1984) Relation between serum sodium concentration and the hemodynamic and clinical responses to converting enzyme inhibition with captopril in severe heart failure. J Am Coll Cardiol 3(4): 1035-1043.
- Hazen SL (2008) Oxidized phospholipids as endogenous pattern recognition ligands in innate immunity. J Biol Chem 283(23): 15527- 15531.
- Virmani R, Burke AP, Kolodgie FD, Farb A (2002) Vulnerable plaque: the pathology of unstable coronary lesions. J Interv Cardiol 15(6): 439- 446.
- Ehara S, Ueda M, Naruko T, Haze K, Itoh A, et al. (2001) Elevated levels of oxidized low density lipoprotein show a positive relationship with the severity of acute coronary syndromes. Circulation 103(15): 1955- 1960.
- Nishi K, Itabe H, Uno M, Kitazato KT, Horiguchi H, et al. (2002) Oxidized LDL in carotid plaques and plasma associates with plaque instability. Arterioscler Thromb Vasc Biol 22(10): 1649-1654.
- Holvoet P, Mertens A, Verhamme P, Bogaerts K, Beyens G, et al. (2001) Circulating oxidized LDL is a useful marker for identifying patients with coronary artery disease. Arterioscler Thromb Vasc Biol 21(5): 844-848.
- Van Berkel TJC, de Rijke YB, Kruijt JK (1991) Different fate in vivo of oxidatively modified low density lipoprotein and acetylated low density lipoprotein in rats: recognition by various scavenger receptors on Kupffer and endothelial liver cells. J Biol Chem 266(4): 2282-2289.
- De Groot PG, Meijers JMC (2011) B2-glycoprotein I: evolution, structure and function. J Thromb Haemost 9(7): 1275-1284.
- Matsuura E, Hughes GRV, Khamashta MA (2008) Oxidation of LDL and its clinical implications. Autoimmun Rev 7(7): 558-566.
- Bartoloni E, Shoenfeld Y, Gerli R (2011) Inflammatory and autoimmune mechanisms in the induction of atheroslcerotic damage in sytemic rheumatic diseases: two faces of the same coin. Arthritis Care Res 63(2): 178-183.
- Matsuura E, Atzeni F, Sarzi-Puttini P, Turiel M, Lopez LR, et al. (2014) Is atherosclerosis an autoimmune disease? BMC Med 12: 47.
- Greco TP, Conti-Kelli AM, Greco T, Doyle R, Matsuura E, et al. (2009) Newer antiphospholipid antibodies predict adverse outcomes in patients with acute coronary syndrome. Am J Clin Pathol 132(4): 613- 620.
- Greco TP, Conti-Kelli AM, Anthony JR, Greco T, Doyle R, et al. (2010) Oxidized-LDL/B2-glycoprotein I complexes are associated with disease severity and increased risk for adverse outcomes in patients with acute coronary syndromes. Am J Clin Pathol 133(5): 737-743.
- Ames PRJ, Ortiz-Cadenas A, Garcia-De La Torre I, Nava A, Oregon- Miranda A, et al. (2010) Rosuvastatin treatment is associated with a decrease of serum oxidized low-density lipoprotein/Beta2- glycoprotein I complex concentration in type 2 diabetes. Br J Diabetes Vasc Dis 10(6): 292-299.
- Sokoll KB, Batuca J, Lopez LR, Hensor E, Emery P, et al. (2014) Effects of Atorvastatin on atherosclerosis and atherogenesis in systemic lupus erythematosus: A pilot study. ISRN Immunology 2014(2014): 295239.
- Kajiwara T, Yasuda T, Matsuura E (2007) Intracellular trafficking of β2-glycoprotein I complexes with lipid vesicles in macrophages: implications on the development of antiphospholipid syndrome. J Autoimmun 29(2-3): 164-173.
- Kuwana M, Matsuura E, Kobayashi K, Okazaki Y, Kaburaki J, et al. (2005) Binding of β2-glycoprotein I to anionic phospholipids facilitates processing and presentation of a cryptic epitope that activates pathogenic autoreactive T cells. Blood 105(4): 1552-1557.
- Shimada K, Mokuno H, Matsunaga E, Miyazaki T, Sumiyoshi K, et al. (2004) Predictive value of circulating oxidized LDL for cardiac events in type 2 diabetic patients with coronary artery disease. Diabetes Care 27(3): 843-844.
- Kraml PJ, Syrovátka P, Potočková J, Anděl M (2013) The oxidized lowdensity lipoprotein/beta2-glycoprotein I complex is associated with abdominal obesity in healthy middle-aged men. Ann Nutr Metab 62(1): 7-13.
- Bliden KP, Singla A, Gesheff MG, Toth PP, Tabrizchi A, et al. (2014) Statin therapy and thromboxane generation in patients with coronary artery disease treated with high-dose aspirin. Thromb Haemost 112(2): 323- 331.
- Berger JS, Rockman CB, Guyer KE, Lopez LR (2014) Pro-atherogenic oxidized low-density lipoprotein/β2-Glycoprotein I (oxLDL/β2GPI) complexes in arterial and venous disease. J Immunol Res 2014: 234316.
- Gurbel PA, Bliden KP, Guyer K, Gesheff MG, Franzese CJ, et al. (2015) OxLDL/β2GPI complex but not free oxLDL is associated with CAD severity in statin naïve patients undergoing elective cardiac catheterization. Journal of the American College of Cardiology 65(10): A1576.
- Song F, Jia W, Yao Y, Hu Y, Lei L, et al. (2007) Oxidative stress, antioxidant status and DNA damage in patients with impaired glucose regulation and newly diagnosed Type 2 diabetes. Clin Sci (Lond) 112(12): 599- 606.
- Schneider MP, Ritt M, Raff U, Ott C, Schmieder RE (2009) Gender is related to alterations of renal endothelial function in type 2 diabetes. Nephrol Dial Transplant 24(11): 3354-3359
- Liao JK (2002) Isoprenoids as mediators of the biological effects of statins. J Clin Invest 110(3): 285-288.
- Essig M, Nguyen G, Prie D, Escoubet B, Sraer JD, et al. (1998) 3-hydroxy- 3-methylglutaryl coenzyme A reductase inhibitors increase fibrinolytic activity in rat aortic endothelial cells: role of geranylgeranylation and Rho proteins. Circ Res 83(7): 683-690.
- Antonopoulos AS, Margaritis M, Shirodaria C, Antoniades C (2012) Translating the effects of statins: from redox regulation to suppression of vascular wall inflammation. Thromb Haemost 108(5): 840-848.
- Deakin S, Guernier S, James RW (2007) Pharmacogenetic interaction between paraoxonase-1 gene promoter polymorphism C-107T and statin. Pharmacogenet Genomics 17(6): 451-457.
- Endres M, Laufs U, Huang Z, Nakamura T, Huang P, et al. (1998) Stroke protection by 3-hydroxy-3-methylglutaryl (HMG)-CoA reductase inhibitors mediated by endothelial nitric oxide synthase. Proc Natl Acad Sci USA 95(15): 8880-8885.
- Laufs U, La Fata V, Plutzky J, Liao JK (1998) Upregulation of endothelial nitric oxide synthase by HMG CoA reductase inhibitors. Circulation 97(12): 1129-1135.
- Blake GJ, Ridker PM (2000) Are statins anti-inflammatory? Curr Control Trials Cardiovasc Med 1(3): 161-165.
- Takemoto M, Liao JK (2001) Pleiotropic effects of 3-hydroxy-3- methylglutaryl coenzyme A reductase inhibitors. Arterioscler Thromb Vasc Biol 21(11): 1712-1719.
- Vasankari T, Ahotupa M, Toikka J, Mikkola J, Irjala K, et al. (2001) Oxidized LDL and thickness of carotid intima-media are associated with coronary atherosclerosis in middle-aged men: lower levels of oxidized LDL with statin therapy. Atherosclerosis 155(2): 403-412.
- Moutzouri E, Liberopoulos EN, Tellis CC, Milionis HJ, Tselepis AD, et al. (2013) Comparison of the effect of simvastatin versus simvastatin/ ezetimibe versus rosuvastatin on markers of inflammation and oxidative stress in subjects with hypercholesterolemia. Atherosclerosis 231(1): 8-14.
- Kim D, Choi SY, Park EH, Lee W, Kang JH, et al. (2012) Nonalcoholic fatty liver disease is associated with coronary artery calcification. Hepatology 56(2): 605-613.
- Bieghs V, Rensen PC, Hofker MH, Shiri-Sverdlov R (2012) NASH and atherosclerosis are two aspects of a shared disease: central role for macrophages. Atherosclerosis 220(2): 287-293.






























