Familial Tracheooesophageal Atresia
Alexandra Anga1* and B. A. Animashaun2
1Division of Neonatology, Department of Paediatrics, Lagos State University Teaching Hospital (LASUTH), Nigeria
2Division of Cardiology, Department of Paediatrics, Lagos State University College of Medicine (LASUCOM), Nigeria
Submission: November 25, 2016; Published: December 08, 2016
*Corresponding author: Alexandra Anga, Division of Neonatology, Department of Paediatrics, Lagos State University Teaching Hospital(LASUTH), Ikeja, Lagos, Nigeria
How to cite this article: Alexandra A, B. A. Animashaun. Familial Tracheooesophageal Atresia. J Cardiol & Cardiovasc Ther. 2016; 2(2): 555586. DOI: 10.19080/JOCCT.2016.02.555586
Introduction
Oesophageal Artesia most times accompanied by tracheoesophageal fistula is not an uncommon clinical condition. It occurs in 1 in 2,500 to 3,000 live births and there is little evidence to support significant geographical or secular variation in the incidence. The majority of cases of Oesophageal Artesia are sporadic/non syndromic. Familial/syndromic cases of oesophageal Atresia are extremely rare representing less than 1% of the total [1]. Survival is directly related to birth weight and to the presence of a major cardiac defect [1]. Infants weighing over 1,500 gram and having no major cardiac problem should have a survival rate of near 100% [1,2].
Case Report
We report a case of a male child born at 41 weeks gestation after an uneventful pregnancy to a 36year old woman, through an uncomplicated Caesarean section. The birth weight was 3,215g. There was however a family history of Oesophageal Atresia with a distal fistula in the baby’s first cousin. The Apgar scores were 9 at one minute and ten at five minutes. Meconium was passed within six hours of delivery. A slight increase in whitish oral secretions was noted. After two hours of life, the baby developed respiratory distress despite the fact that he had not yet been fed. The respiratory rate was 96 breaths per minute; there was reduced intensity of breath sounds at the lung bases and generalised rhonchi.
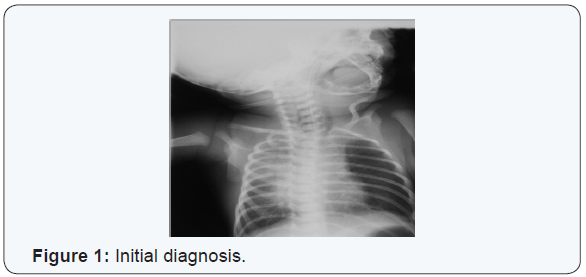

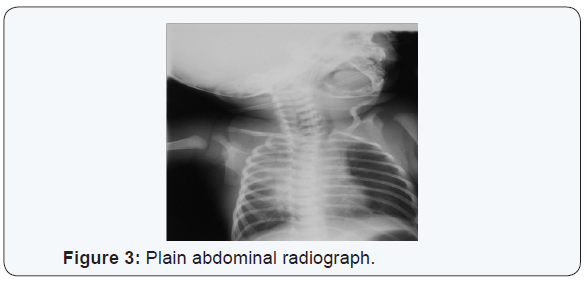
An initial diagnosis was made of aspiration pneumonitis (Figure 1). After an initial turbulent period the baby’s clinical condition improved. On the fourth day of life repeated attempts to pass a nasogastric tube for feeding failed. Intraoesophageal suction continued to yield copious aspirates. A tube containing light barium was passed and a plain radiograph of the neck and chest taken (Figure 2). This demonstrated a blind proximal oesophageal pouch. A plain abdominal radiograph showed abundant gastric and intestinal gas (Figure 3). Bronchoscopy subsequently confirmed oesophageal Atresia. Clinical evaluation and an echocardiogram excluded congenital heart disease. The baby underwent primary surgical repair on the eighth day of life and has since recovered.
Discussion
Oesophageal Atresia is a relatively common congenital abnormality. Failure to diagnose the condition antenatally puts the patient at risk of aspiration. At our centre in 2007 there were 3,387 live births among who were three cases of oesophageal Atresia. There is a paucity of local data however to compare with. The index case fits the most commonly described (86%) variety of tracheooesophageal malformations in which there is a blind proximal oesophageal pouch and a distal fistulous connection with the trachea. Tracheooesophageal malformations are often associated with other congenital abnormalities mostly cardiac like VSD, PDA or Fallots Tetralogy and may be a component of VATER association.
The presence of an associated defect negatively impacts on the outcome. Our patient did not have any congenital cardiac abnormality. One major feature of interest in the index case is the fact that a paternal first cousin also had tracheo-oesohageal fistula. This underlines the fact that although most cases are sporadic, familial cases do exist [3], we wonder if this could be a case of familial Tracheoesophageal fistula. The overall data about Oesophageal atresia in twins reveal discordance in the great majority with a concordance rate as low as 2.5% [4]. The recurrence risk of the anomaly in siblings of an affected child calculated from data from familial studies is comparable with a recurrence risk of a multifactorial condition (1%). And the distribution of the anomaly in first and second degree relatives does not fit into a monogenic mode [3].
Conclusion
These data suggest that in most cases aetiological factors are non genetic. No pedigree so far has been demonstrated with direct transmission over three generations [2]. There are however, well-defined instances where genetic factors clearly play a role [1]. Recently three genes have been identified in humans with a role in OA. Feingold syndrome (N-MYC), Anopthalmia – OA (AEG) syndrome (SOX2) and CHARGE syndrome (CHDY). This is of interest and should lead to greater insight into the aetiology of this condition. The outcome of babies with Oesophageal atresia and TOF has significantly improved over the last decade [4].
Prompt recognition and timely surgical intervention leads to a good outcome in babies of weights above 1500 grammas and the absence of cardiac and other associated anomalies. It should be noted that fifty per cent of affected babies will have an associated malformation and effort should be made to detect these as soon as possible [1,2,4]. Oesophageal Atresia with TOF remains a prime consideration in a neonate who develops feeding difficulties and respiratory distress in the first few days of life.
Prompt recognition, appropriate clinical management to prevent aspiration and swift referral to an appropriate tertiary specialist centre will result in a significant improvement in rates of morbidity and mortality. Antenatal diagnosis however allows the delivery centre to be optimised thus improving the outcome. Improvement in the survival of high risk cases is related to better post operative care [6] Long term follow up is essential because of the ongoing morbidity of the patients [5-12].
References
- Lewis Spitz (2007) Oesophageal atresia.Orphanet Journal of Rare Diseases 2: 24.
- Goyal A, Jones MO, Couriel JM, Losty PD (2009) Oesophageal atresia and tracheo-oesophageal fistula. Arch Dis Child Fetal Neonatal Ed 91(5):F381-F384.
- Van Staey M, De Bie S, Matton MT, De Roose J (1984) Familial congenital esophageal atresia. Personal case report and review of the literature. Hum Genet 66(2-3): 260-266.
- Shaw-Smith C (2006) Oesophageal atresia, tracheo-oesophageal fistula, and the VACTERL association: review of genetics and epidemiology. J Med Genet 43(7): 545-554.
- Engum SA, Grosfeld JL, West KW, Rescorla FJ, Scherer LR 3rd (1995) Analysis of morbidity and mortality in 227 cases of esophageal atresia and/or tracheoesophageal fistula over two decades.Arch Surg 130(5):502-508.
- Rawi AL, Booker PD (2007) Oesophageal Atresia and Tracheoesophageal fistula Continuing Education in Anaesthesia, critical care and pain (1): 15-19.
- Kovesi T, Rubin S (2004) Long-term complications of congenital esophageal atresia and/or tracheoesophageal fistula. Chest 126(3): 915-925.
- Agrawal L, Beardsmore CS, Macfadyen UM (1999) Respiratory function in childhood following repair of Tracheooesophageal Atresia and Tracheooesophageal fistula. Arch Dis Child 81(5): 404-408.
- Gordon M.Wyant, Cram Robert W (1963) The management of infants with Tracheoesophageal fistula. Canadian Journal of Anaesthesia 10: 93-102.
- Maria Cristina Digiho, Bruno Mariano Pietro Bogolan, Aldo Giannotti, Bruno Dallapiccola (1999) Microdeletion 22q11 and Oesophageal Atresia. Journal of Medical Genetics 36: 137-139.
- J.O Adeniran, T O Odebode (2005) Congenital Malfomations in Paediatric and Neurosurgical Practices:Problems and Pattern. Sahel Medical Journal January- March 8(1).
- M Mukhtar-Yola, M IBRAHIM, R Belonwu, Z Farouk, A Mohammed (2005) The Prevalence and Outcome of Obvious Congenital Malformation among Inborn Babies at Aminu Kanu Teaching Hospital, Kano. Nigerian Journal of Paediatrics 32(2): 47-51.






























