Impact of Cardiac Resynchronization Therapy on Cardiac Electric Remodeling and its Clinical and Echocardiographic Correlation
Ahmed Abdelmoneium, Eman EL keshk, Shaimaa A. Mostafa* and Haytham Ramadan
Cardiovascular medicine department, Benha university hospital, Egypt
Submission: December 18, 2015; Published: January 05, 2016
*Corresponding author: Shaimaa A. Mostafa, Benha faculty of medicine, Benha university hospital, cardiovascular medicine department, Egypt, Tel: 01229122843; Email: shaimaamustafa2011@gmail.com
How to cite this article: Abdelmoneium A, Eman El K, Mostafa S A, Haytham R. Impact of Cardiac Resynchronization Therapy on Cardiac Electric Remodeling and its Clinical and Echocardiographic Correlation. J Cardiol & Cardiovasc Ther. 2015; 1(2): 555557. DOI: 10.19080/JOCCT.2016.01.555557
Abstract
Aim: impact of cardiac resynchronization therapy on cardiac electric remodeling and its clinical and echocardiographic correlation.
Patients and Methods:50 patients candidate for CRT were included and assessed before and 3 months after implantation by history taking, clinical examination, transthoracic echocardiogram, ECG and six minutes walk test.
Results:50 patients (35 males and 15 females, mean age 54.88±7.48 years) with implanted CRT. All patients were in sinus rhythm, QRS duration ≥120 ms and LBBB. There was clinical response to CRT as NYHA class improved p<0.001 and 6 min walk test p<0.001, electrocardiographic response where apart from RR interval, there were significant differences in QRSD, QT, QTc, QTd, and TPE between pre and post CRT measurements (P<0.001) and echocardiographic response as LV volume improved in 78%, EF in 14% and change (ΔQRSD) significantly correlated with changes in NYHA, LVEDD, LVESD, and LVEF. (ΔQTd) significantly correlated with change in NYHA but not with echocardiographic changes. But ( ΔTPE) significantly correlated with LVEDD, LVESD, LVEDV, LVESV, and LVEF but not with ΔNYHA. Area under the curve was 0.813 for ΔQRSD (p=0.005), 0.56 for ΔQT (p=0.56), 0.59 for ΔQTc (p=0.41), 0.823 for ΔQTd (p=0.004), and 0.8 for ΔTPE (p=0.007). ΔQTd of ≥-20 ms had highest sensitivity for predicting both clinical and Echocardiographic response, while ΔQRSD of ≥-20 ms had highest specificity.
Conclusion:CRT induced reverse electric remodeling including depolarization and repolarization changes on unpaced ECG that was associated with reverse structural remodeling and clinical improvement.
Introduction
Heart Failure (HF) represents approximately 1–2% of the adult population in developed countries with the prevalence rising to ≥10% among persons70 years of age or older [1]. There are many causes of HF; however the most common cause of HF is coronary artery disease [2]. What characterizes untreated systolic dysfunction is progressive worsening of these changes over time, with increasing enlargement of the left ventricle and decline in EF, even though the patient may be symptomless initially. The goals of treatment in patients with established HF are to relieve symptoms and signs, prevent hospital admission and improve survival [3].
The idea of CRT is based on the concept of improving dyssynchronus conduction of the heart by biventricular pacing which leads to a significant alleviation of symptoms and increase in exercise capacity. On average, NYHA function class decreased by 0.5–0.8 points, the 6 min walk distance increased by 20%, and peak oxygen consumption increased by 10–15%. The functional benefits and quality of life improvements were sustained [4].
A consistent finding in the randomized trials designed with up to 6 months of follow-up has been an up to 15% absolute reduction in LV end-diastolic diameter and up to 6% increase in LVEF following CRT [2]. REVERSE trial showed a significant degree of reverse LV remodeling was observed among the patients assigned to CRT, manifested by decreases in the LV end systolic and diastolic volumes and an increase in LVEF [2]. Non responders to CRT (defined as patients who doesn’t show decrease in NYHA classification more than 1 within 3 months after CRT) have been defined in one third of the patients [5].
Electric remolding in HF occurs due to left ventricular dyssynchrony and it can be predicted by the following: QRS duration: in patients with HF, left ventricular electrical dyssynchrony is associated with QRS prolongation, it would predict the mechanical dyssynchrony therefore can predict the response to CRT based on the idea of biventricular pacing will decrease the left ventricle dyssynchrony thus will decrease QRS duration [6].
QT interval, QT dispersion & QTc
They are considered to be sensitive indicator of left ventricular dyssynchrony. We can assume that QT dispersion becomes longer when cardiac pathological changes occur causing left ventricular dyssynchrony and will be improved after CRT, thus can be a good predictor of reverse electrical remodeling following CRT [7].
T wave morphology & T peak –T end dispersion
T wave amplitude variability (TAV) can be a promising non invasive predictor of arrhythmogenic events in patients with cardiomyopathy. The decrease in TAV after CRT is associated with left ventricular reverse remodeling and it indicates reduction of the arrhythmogenic event, so it can be of good predicting value for patients follow up after CRT [7].
Aim
Impact of cardiac resynchronization therapy on electric remodeling of the heart and its clinical and echocardiographic correlation.
Patients and Methods
The study was conducted on fifty patients who are candidate for CRT at National Heart Institute in the period from October 2014 to June 2015. All patients had CRT-P devices (St. Jude) and were assessed before and at least three months after implantation
Inclusion criteria:
All patients fulfilled all the following criteria:
- New York Heart Association (NHYA) functional class III or ambulatory class IV in heamodynamically stable condition for at least 4 weeks despite optimal medical treatment.
- Left ventricular ejection fraction (LVEF) less than or equal to 35% as assessed by echocardiography.
- Intraventricular conduction delay defined by a QRS duration ≥ 120 m sec if LBBB or >140msec if non LBBB.
- All patients were on optimal recommended pharmacological therapy including (ACE inhibitors or ARBS, B blockers, Aldosterone antagonists and diuretic).
Exclusion criteria:
- NYHA class I or II.
- Narrow QRS complex.
- Ejection fraction more than 35%.
- Presence of atrial fibrillation.
- Amiodarone therapy.
- Device malfunctions.
Each patient was subjected to history taking, clinical examination, transthoracic echocardiogram, 12 lead surface ECG and six minutes walk test before implantation and followed by reevaluation at least three months after implantation. ECGs were done with CRT off mode to acquire native electrocardiograms then Echocardiography and six minutes walk test were done for every patient to assess response.
Investigations
ECG acquisition and analysis:
Standard 12-lead surface ECGs with a paper speed of 25 mm/s and 10 mm/mV gain were analyzes prior to and at least three months after implantation while setting the device to CRT-off mode. CRT-on mode was restored after acquiring ECGs. Measurements were assessed manually and taken from the average of 2 measurements made by 2 Cardiologists who were blinded to each other’s measurements and to patients’ data. The following measurements were taken:
- PR interval (normally 0.12 to 0.20 sec)
- QT interval measured from the beginning of QRS to the end of the T-wave defined as the point of return to the isoelectric line. QTc interval was calculated by Bazett’s formula (QTc=QT/square root of RR interval in seconds). Intervals were measured in the lead with the highest T wave amplitude among leads II, V5, or V6, using the same lead in pre and post-CRT ECGs (The normal value for the QTc in men is ≤0.44 sec and in women is ≤0.45 sec).
- QT dispersion (QTd), defined as the difference between the longest and shortest QT interval among all lead ( normal values from 10 to 71 ms)
- T wave peak to end (TPE) duration, defined as the duration from the peak to the end of T wave in II, V5, V6 according to the highest T wave amplitude, using the same lead in pre and post-CRT ECGs.
Intrinsic QRS duration (QRSD) and axis (The entire QRS duration normally lasts from 0.06 to 0.10 seconds) .
Echocardiography
Trans-thoracic echocardiographic examination with machine-integrated ECG recording was performed, mostly with the patients lying in the left lateral decubitus position, using a Vivid 5 Dimension machine with an M4S matrix sector array probe with a frequency range of 1.5 to 3.6 MHz (GE Vingmed Ultrasound, Horten, Norway Left ventricular EF, dimensions and volumes (LVEDV & LVESV) were measured utilizing modified Simpson’s equation (The endocardium of the apical two and four chamber echocardiographic views in systole and diastole is traced manually.
Wall motion abnormality at rest and was assessed for patients by dividing the left ventricle into 16 segment and assessment of each segment according to the American Society of Echocardiographys as follow;
- normal
- hypokinesis
- akinesis
- dyskinesis
Mitral regurgitation
Degree of mitral regurge were assessed before and after CRT implantation according to The 2003 American Society of Echocardiography’s consensus statement on echocardiographic quantification of valvular regurgitation and classified into (mild to moderate, moderate to severe mitral regurgitation according to jet area measurement as follow Mild < 4 cm, moderate 4-8cm and sever>8cm).
Left atrium Diameter
Was assessed for every patient pre and at least three months after CRT implantation in the PLAX view. Pulmonary artery systolic pressure: for diagnosis of Pulmonary hypertension PASP was estimated from the tricuspid regurgitant jet velocity using the modified Bernoulli equation and adding the estimated right atrial pressure (RAP) on the basis of inferior vena cava (IVC) diameter and collapsibility as follow IVC ≤2 cm and >50% collapsibility, 5 mm Hg; IVC >2 cm and ≥50% collapsibility, 10 mm Hg; IVC >2 cm and <50% collapsibility, 15 mm Hg; and IVC >2 cm and no collapsibility, 20 mm Hg. We assumed RAP 10 mm Hg for cases with IVC ≤2 cm and ≤50% collapsibility.
6 min walk distance
Which is a simple objective measurement to assess patient’s functional capacity.
Technique:
- Flat, straight corridor 30 m (100 feet) in length.
- Turnaround points marked with a cone.
- Patient should wear comfortable clothes and shoes.
- Patient rests in chair for at least 10 minutes prior to the test (ie, no warm -up period)
- Record baseline heart rate and blood pressure.
- Instruct the patient to walk as far as possible for 6 minutes.
- At the end of the test, the spot where the patient stopped was marked at the floor .
- The distance walked was recorded and calculated.(Borg GA.1982)
- The test was performed to each patient before and at least three months after device implantation.
Implantation data: Pacing leads were inserted through standard subclavian vein approach. The RA lead was placed in RA appendage, The RV lead tip was placed in the apex of the right ventricle. The LV lead tip was placed in the posterolateral cardiac vein when possible or in an alternative posterior or lateral vein. The location chosen for LV lead was that giving the greatest spatial separation from the tip of the RV lead, with stable LV capture and without diaphragmatic capture at four times threshold voltage .Device programming was done during follow up of the patients in the outpatient clinic to assess lead impedances, pacing, sensing thresholds, optimization of AV and VV delays and detection of device malfunction.
Implantation data:
Complications of CRT implantation include coronary sinus or coronary vein trauma, pneumothorax, diaphragmatic/phrenic nerve pacing, and infection were assessed for all patient.
Criteria of CRT Response:
Response to CRT can be divided into 2 main categories: clinical end points indicating improved clinical status (NYHA functional class by ≥1 NYHA class, quality-of-life, exercise capacity expressed as 6-min walking distance) and Echocardiographic end points indicating improved LV systolic function or reversed LV remodeling (decrease in LV end systolic volume by ≥15%) [6].
Electrocardiograic predictor to clinical and Echocardiographic response is defined as decrease in intrinsic QRS duration by ≥20 ms [8].
Statistical Analysise
Continous variables were expressed as mean ± Standard deviation. Paired and unpaired and Student’s t-test and nonparametric tests were used for comparisons between baseline and follow up.
Categorical variable were expressed as numbers or percentages were analyzed with the chi-square test. D’Agostino- Pearson test for normal distribution was done and accordingly, non parametric test was chosen when distribution was nonnormal. Comparison between continuous data before and after CRT was done using paired t-test. Comparison between continuous data in responders and non responders was done using unpaired t-test or Mann-Whitney test as appropriate. Receiver operating characteristic (ROC) analysis were carried out to examine the predictive ability of ECG parameters to the response to CRT and to determine cut-off values, sensitivity and specificity for each ΔiQRS duration .The area under curve (AUC) provided a measure of the overall discriminative ability of a model.
Correlations were made using Pearson correlation coefficient or Spearman rho as appropriate. Risk assessment for ECG parameter was expressed by unadjusted odds ratio after dichotomization according to the cut off values. P value was considered significant if < 0.05.
Results
This single center, prospective study included 50 patients (35 males and 15 females, with mean age of 54.88±7.48 years) with implanted CRT device in the period from October 2014 to June 2015. All patients were assessed twice at the time of implantation and 3 months after. All patients were in sinus rhythm, with baseline QRS duration ≥120 ms and LBBB morphology. All programmable data were accepted with ≥98% biventricular pacing.
Demographic data of the studied patients
The study included 50 patients 35 males (70%) and 15 females (30%), with mean age of 54.88±7.48. In 50% of the patients the underling etiology was ICM and the other 50% was DCM.
II. Risk factors: 24patients (48%) were hypertensive, 22 patients (44%) had diabetes mellitus, 30 patients (60%) had dyslipidemia and 10 patients (20%) were smokers.
Clinical response to CRT
NYHA class
20 patient (40%) improved from NYHA class (III →I ), 8 patients (16%) improved from NYHA class (III →II ), 8 patients (16%) improved from NYHA class (IV →I ),4 patients (8%) improved from NYHA class (IV →II ) and 10 patients (20%) remained in the same NYHA class 3 months after CRT (Table 1).
Results of Chi-square test showed that before CRT implantation72% of the patients were NYHA class III and 28% were NYHA class IV after CRT 58% were class I, 24% were class II, 16% were class III and 2% were class IV with p value< 0.001 (Table 1).

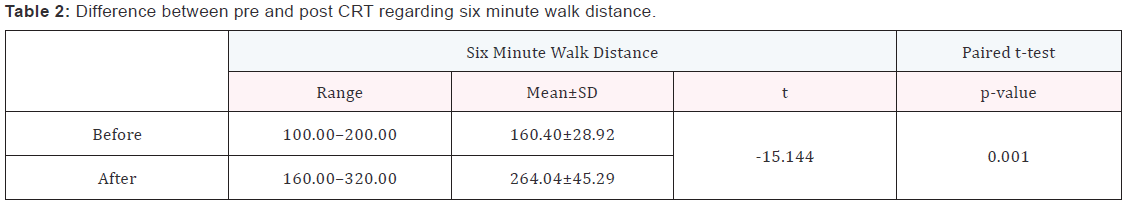
Six minutes walk test
- The mean 6MHW distance at baseline was 160.40 + 28.92 meters reaching 264.04 ± 45.29 meters 3 months after CRT implantation (P value 0.001) (Table 2).
- The improvement in the distance was detected in 40 cases who are clinical responders that represent about 80% of studied cases (Table 2).
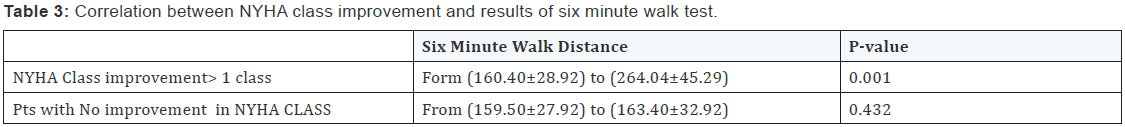
- There was statistically significant correlation between NYHA class improvement > 1 class and six minute walk distance p=0.001 (Table 3).
Electrocardiographic response to CRT:
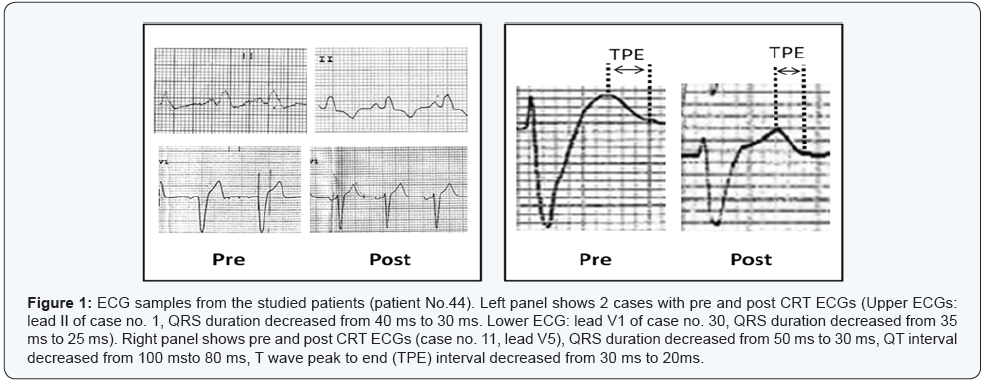
All ECG parameters were taken without pacing (with CRT off mode) 3m after CRT implantation. Apart from RR interval, there were highly significant differences in QRSD, QT, QTc, QTd, and TPE between pre and post CRT measurements (P<0.001) (Table 4 & Figure 1).
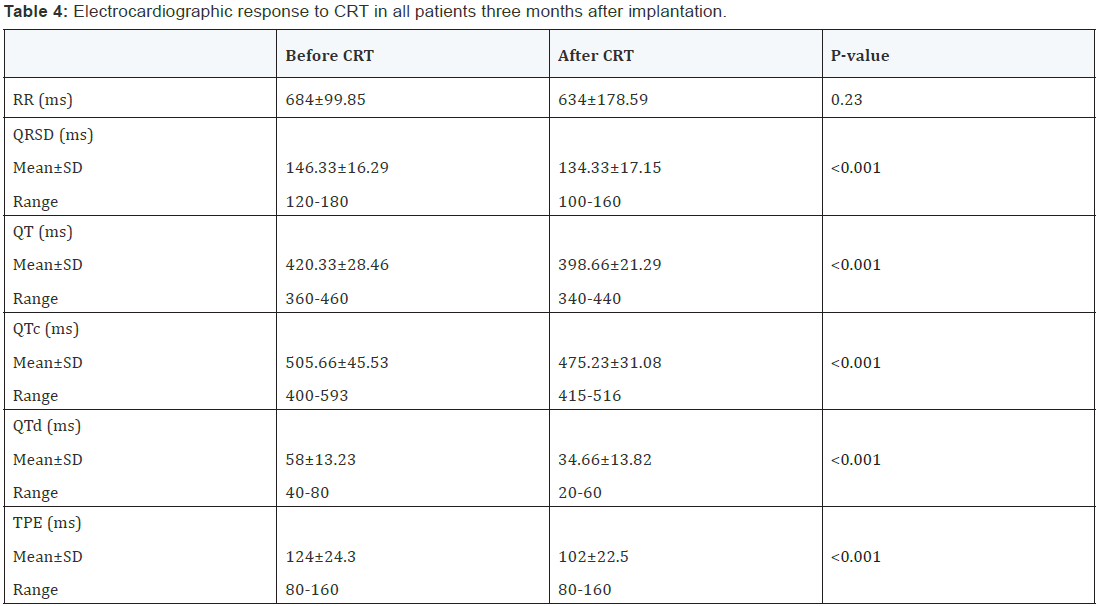
Mean change in QRSD (ΔQRSD) was -12±9.96 ms (median -20 ms). ΔQT was -21.33±19.07 ms (median -20 ms). ΔQTc was -30.45±36.03 ms (median -23.5 ms). ΔQTd was -23.33±15.82 ms (median -20 ms). ΔTPE was -22±22.5 ms (median -20 ms).
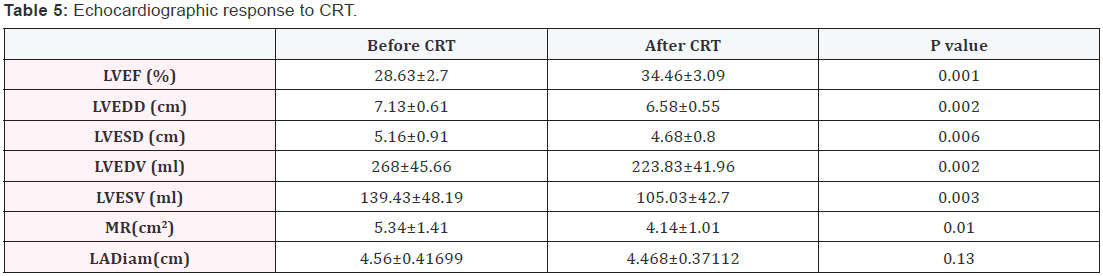
Echocardiographic response to CRT
- Regarding left ventricular volume 39 patients improved representing 78% of study cases.
- Regarding left ventricular ejection fraction patients with significant improvement with>10% increase in EF were 7 patients representing 14% of study cases.
- There was statistically significant difference in LVESV before and after CRT. P value was 0.003.
- There was statistically significant difference in LVEDV before and after CRT. P value was 0.002 (Table 5).
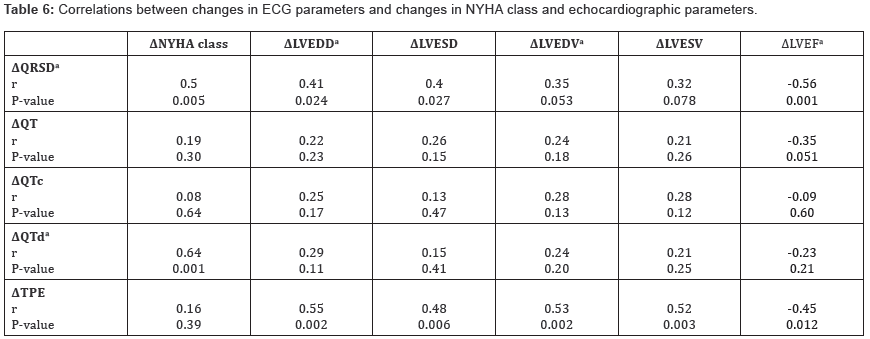
Correlations between changes in ECG parameters and changes in NYHA class and echocardiographic parameters
Change in native QRSD (ΔQRSD) was significantly correlated with changes in NYHA class, LVEDD, LVESD, and LVEF. (ΔQTd) was significantly correlated with change in NYHA class but did not correlate with any of the echocardiographic changes. On the other hand, (ΔTPE) was significantly correlated with changes in LVEDD, LVESD, LVEDV, LVESV, and LVEF but did not correlate with ΔNYHA class (Table 6).
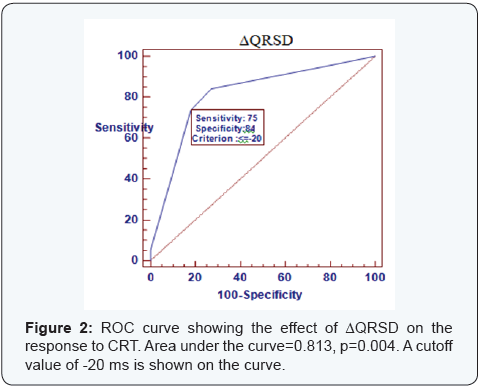
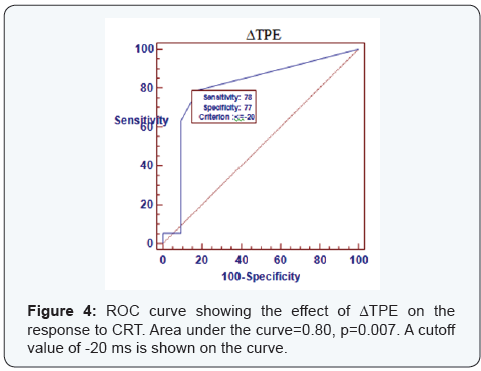
ROC curve
ROC curve analysis was done to find the best cutoff values for the changes in ECG parameters associated with the clinical and Echocardiographic response to CRT (Figures 2-4). Area under the curve was 0.813 for ΔQRSD (p=0.005), 0.56 for ΔQT (p=0.56), 0.59 for ΔQTc (p=0.41), 0.823 for ΔQTd (p=0.004), and 0.8 for ΔTPE (p=0.007). ΔQTd of ≥-20 ms had the highest sensitivity for predicting both clinical and Echocardiographic response, while ΔQRSD of ≥-20 ms had the highest specificity.
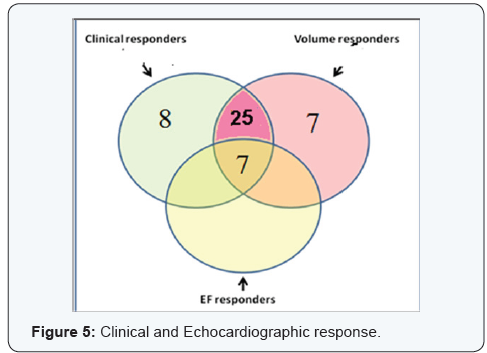
Both clinical and Echocardiographic response occurred in 32 patients (64 %) who were considered as CRT responders. Clinical response without criteria of Echocardiographic response was found in 8 patients (16 %), while Echocardiographic response without clinical improvement was found in 7 patients (14 %) (Figure 5).

Complications related to CRT implantation
Patients who had immediate complications after implantation of CRT represented (0.8%): 2 patients had hematomas at site of implantation representing (0.4%), 2 had diaphragmatic pacing representing (0.4%).
Discussion
Cardiac resynchronization therapy has been shown to improve heart failure, reduces hospitalization and improves the status of LV dysfunction. However, a number of patients remain unresponsive to therapy. The cause of unresponsiveness has been an issue of research in many trials and has been a concern in follow up of CRT patients [6].
Electric remodeling in HF is associated with adverse clinical outcome. Widening of the QRS complex is associated with mechanical dyssynchrony, while prolongation of repolarization in associated with ventricular arrhythmias and sudden cardiac death [3].
The main focus of this study was to evaluate the impact of Cardiac Resynchronization Therapy on electrical remodeling of the heart and its clinical and Echocardiographic correlation after three months of implantation.
In this study there were significant changes in both depolarization and repolarization parameters on surface ECG after CRT. Also there were significant differences between responders and non responders regarding changes in QRSD, QTd, and TPE. Furthermore, these changes were significantly correlated with changes in NYHA class and echocardiographic parameters. Changes in QRS duration (ΔQRSD) and repolarization heterogeneity (ΔQTd and ΔTPE) could predict response to CRT with high sensitivity and specificity [9].
Regarding CRT response
In our study, 8 patients had clinical improvement without criteria of echocardiographic response, which might be due to more patient compliance to therapy or may be placebo effect. Therefore, correlations with ECG parameters were done only in patients with both clinical and echocardiographic response who were defined as CRT responders. Response to CRT occurred in 32/50 patients (64 %), which is similar to the generally reported rate of response [10]. All CRT responders in this study had ≥15% reduction in LVESV which is considered the hallmark of LV reverse remodeling [11]. Hence, the occurrence of ECG changes in responders indicates association between reverse electrical and structural remodeling following CRT.
Regarding RR interval
There was no significant change in RR interval on the ECG after CRT. This agrees with the studies of Henrikson and Sebag [9] who reported similar observations [9]. The pretreatment with β blocker and/or ivabradine by the majority of patients who implanted CRT may explain this finding.
Regarding QRS duration: In our study, native QRSD decreased significantly after CRT (from 146.33±16.29 to 134.33±17.15 ms, p<0.001). This may indicate partial recovery of left bundle branch conduction. Sebag et al. [12] reported similar findings in 85 patients who were evaluated after 1 year of CRT (intrinsic QRS decreased from 168.0 ± 19.7 to 149.6 ± 31.6 ms, p < 0.0001). They considered patients with decrease in intrinsic QRSD by>- 20 ms as “electrocardiographic responders”, who had greater rate of clinical (p=0.035) and echocardiographic (p=0.023) response [12].
Another study of Mischke et al. [13] showed that there was significant decrease in native QRSD one year after CRT device implantation [13].
Also Dizon et al. [14] reported a case with loss of LBBB and complete normalization of QRS complex after CRT [14].
In contrast to these findings of our study, Stockburger et al. [15] did not find any decrease in unpaced QRSD after CRT in a series of 21 patients. This controversy may be explained by:
- About one third of their patients were on amiodarone
which influences intraventricular conduction,
The different methods of ECG interval measurement (Stockburger et al. [15] relied on computed measurement, while our study relied on manual method)
- The variable duration in HF before CRT; longer duration might be associated with irreversible fibrotic changes [15].
Our study observed that ΔQRSD was significantly larger in responders than non responders. ΔQRSD also correlated significantly with changes in NYHA class, LV diameters, and LVEF. It was found that a ΔQSRD of ≥-20 ms could predict response to CRT (p=0.003) with 75% sensitivity and 84% specificity. Similarly, [12] reported that a ΔQRSD of ≥-20 ms was optimal for predicting both clinical and echocardiographic response. Since QRSD is well known as a predictor of total mortality and sudden cardiac death in patients with HF [16], the decrease in native QRSD after CRT may affect prognosis [16].
Tereshchenko et al. [17] reported that reduction of native QRS complex by -10 ms after CRT was associated with a fourfold decrease in the risk of death or sustained ventricular tachycardia [17].
Regarding QT and QT dispersion
In heart failure patients, the altered ion channel dynamics result in prolongation and heterogeneity of repolarization. In our study, there were significant reductions in native QT, QTc, and QT dispersion (QTd) after CRT. We did not calculate the corrected QTd (QTc dispersion) as it was previously reported that QTd is independent of heart rate [18]. Unlike ΔQT and ΔQTc, ΔQTd was significantly larger in responders than non responders, and correlated significantly with ΔNYHA class. We found that a ΔQTd of ≥-20 ms could predict response to CRT (p<0.001) with sensitivity of 100% and specificity of 67%. In contrast to these findings; Henrikson et al. [9] and Sebag et al. [12] found no significant changes in native QT and QTc after CRT. However, since they did not exclude patients on antiarrhythmic drugs, their assessment of repolarization could not be relied upon. Despite the significant reductions in QT and QTc intervals after CRT in our study, they did not predict response to CRT [9,12].
Regarding to T wave peak to end (TPE) interval
The peak of T wave coincides with epicardial repolarization, while the end of T wave coincides with endocardial repolarization [19]. In heart failure patients, the preferential prolongation of the M cell action potential results in a transmural dispersion of repolarization (TDR), which can be estimated from the ECG as the interval between the peak to the end of the T wave [20]. TPE interval may be less dependent on cardiac depolarization changes than QT measurements, and may therefore provide a more reliable estimation of ventricular dispersion of repolarization in patients with wide QRS complex [21]. In 1081 healthy subjects, reference value of TPE interval was 94 ms in men and 92 ms in women and was independent of heart rate, therefore, does not need to be corrected [22].
In our study, baseline mean TPE interval was higher than normal (124±24.3 ms), which significantly decreased after CRT. Previous studies reported that TPE prolongation was independently associated with increased risk of sudden cardiac death in cardiovascular disease [21], but not in healthy population [23]. The Reduction of TPE interval may indicate partial reversal of the adverse electric remodeling associated with heart failure. Furthermore, ΔTPE was higher in responders than non responders and correlated significantly with changes in all echocardiographic parameters that indicate reverse structural remodeling. We found that a ΔTPE of ≥-20 ms could predict response to CRT (p=0.001) with sensitivity of 78% and specificity of 77%. To the best of our knowledge, there are no previous data regarding the predictive value of changes in native repolarization heterogeneity on surface ECG after CRT.
In our study 20 patient (40%) improved from NYHA class(III to I) , 8 patient(16%) improved from NYHA class (III to II),8 patient (16%) improved from NYHA class(IV to I),4 patient (8%) improved from NYHA class (IV to II) and 10 patient (20%) remained in the same NYHA classes 3 months after CRT implantation.
The results of our study are in agreement with Ypenburg et al. [24] they studied 302 patients with NYHA class III-IV and LVEF ≤ 35% with a wide QRS complex, to define the rate of responders and reverse remodeling for follow up of 6 months after CRT. About 57% of the patients improved in one NYHA functional class and 12% showed an improvement of two NYHA functional classes while 31% remained unchanged at 6 months follow-up after CRT. The reason for unresponsiveness to CRT in this study was attributed to presence of a large percentage of ischemic patients in the whole study population which accounted for 58% compared to non ischemic whom accounted for the rest of the study population [24].
The result of study of Stellbrink et al. [25] was in concordant to our study as they studied 25 patients with advanced heart failure for follow up 6 months after CRT implantation with echocardiographic measurements of both dimensions and volumes. The aim of the study was to investigate whether CRT leads to long-term improvement in LV diameters and reduces LV volumes in patients with HF and whether baseline echocardiographic variables were predictive of long-term improvement after CRT. In Stellbrink et al. [25] study the mean LVEDD and LVESD were significantly reduced after CRT. The mean LVEDD was reduced from 71 ± 10 to 68 ±11 mm and LVESD was reduced from 63 ± 11 mm to 58 ±11 mm respectively [25].
The author concluded that spherical shape in dilated hearts was associated with increased wall stress, and that a change in LV sphericity have been correlated to an increase in exercise capacity and may involve a reduction in regional wall stress, myocardial oxygen demand and functional MR [25].
In our study, there was statistically significant difference as regards to 6 MHW distance in clinical responders with NYHA class improvement ≥one class shows difference in the distance walked Form (160.40 ± 28.92) to (264.04 ± 45.29) three months post implantation with p value (0.001). On the other hand patients who develop no improvement in NYHA class show modest increase in distance that range from (159.50±27.92) to (163.40 ± 32.92) with p value (0.432) it means that clinical responders shows corresponding increase in six minute walk test coinciding with functional class improvement. The study of Yu CM [10] was in concordant to our study .they enrolled Twenty-five patients with NYHA class III to IV heart failure and electrocardiographic wave complex duration>140 ms receiving biventricular pacing therapy were assessed serially up to 3 months after pacing, Echocardiography, six minutes walk test were performed . Echocardiography show improvement in left ventricular (LV) end-diastolic (205+/-68 versus 168+/-67 mL, P<0.01) and end-systolic volume (162+/-54 versus 122+/-42 mL, P<0.01); and improved 6-minute walk distance after pacing for 3 months that was considered objective measurement of clinical improvement [10].
References
- Mostard A, Hoes AW (2007) Clinical epidemiology of heart failure. Heart 93(9): 1137-1146.
- Ackerman MJ, Priori SG, Willems S, Berul C, Brugada R, et al. (2011) HRS\EHRA expert consensus statement on the state of genetic testing for the chanalopathies and cardiomyopathies: this document was developed as partnerships between the Heart Rhythm Society (HRS) and the European Heart Rhythm Association (EHRA). Heart Rhythm 8(8): 1308-1339.
- Masson S, Latini R, Anand I, Barlera S, Angelica L, et al. (2008) Prognostic value of changes in N-terminal pro-brain naturitic peptidein Val-HeFT (Valsartan Heart Failure trial). J Am Coll Cardiol 52(12): 997-1003.
- McMurray J (2010) Clinical practice. Systolic heart failure. N Engl J Med 362(3): 228-238.
- Yu C, Fung W, Lin H, Zhang Q, Sanderson J, et al. (2003) Predictors of left ventricular reverse remodeling after cardiac resynchronization therapy for heart failure secondary to idiopathic dilated or ischemic cardiomyopathy. Am J Cardiol 91(6): 684-688.
- Bleeker G, Schalij M, Molhoek S, Verwey H, Holman E, et al. (2004) Relationship between QRS duration and left ventricular dyssynchrony in patients with end-stage heart failure. J Cardiovasc Electrophysiol 15(5): 544-549.
- Kountouris E, Pappa E, Korantzopoulos P, Pappas K, Karanikis P, et al. (2004) Usefulness of predischarge exercise electrocardio-graphic testing in detecting the late patency status of the infarct-related artery. Heart Vessels 19(3): 111-115.
- Ypenburg C, van Bommel RJ, Borleffs CJW, Bleeker GB, Boersma E, et al. (2009) Long-term prognosis after cardiac resynchronization therapy is related to the extent of left ventricular reverse remodeling at midterm follow-up. J Am Coll Cardiol 53(6): 483-490.
- Henrikson CA, Spragg DD, Cheng A, Capps M, Devaughn K, et al. (2007) Evidence for electrical remodeling of the native conduction system with cardiac resynchronization therapy. Pacing Clin Electrophysiol 30(5): 591-595.
- Yu CM, Hayes DL (2013) Cardiac Resynchronization Therapy. Eur Heart J 34(19): 1396-1403.
- St John Sutton M, Ghio S, Plappert T, Tavazzi L, Scelsi L, et al. (2009) Resynchronization reverses Remodeling in Systolic left ventricular dysfunction (REVERSE) Study Group. Cardiac resynchronization induces major structural and functional reverse remodeling in patients with New York Heart Association class I/II heart failure. Circulation 120(19): 1858-1865.
- Sebag FA, Martins RP, Defaye P, Hidden-Lucet F, Mabo P, et al. (2012) Reverse Electrical Remodeling by Cardiac Resynchronization Therapy: Prevalence and Clinical Impact. J Cardiovasc Electrophysiol 23(11): 1219-1227.
- Mischke K, Knackstedt C, FacheK, Reith S, Rana O, et al. (2011) Electrical remodelling in cardiac resynchronization therapy: Decrease in intrinsic QRS duration. Acta Cardiol 66(2): 175-180.
- Dizon J, Horn E, Neglia J, Medina N, Garan H (2004) Loss of left bundle branch block following biventricular pacing therapy for heart failure: evidence for electrical remodelling? J Interv Card Electrophysiol 10(1): 47-50.
- Stockburger M, Nitardy A, Fateh-Moghadam S, Krebs A, Celebi O, et al. (2008) Electrical remodeling and cardiac dimensions in patients treated by cardiac resynchronization and heart failure controls. Pacing Clin Electrophysiol 31(1): 70-77.
- Baldasseroni S, Gentile A, Gorini M, Marchionni N, Marini M, et al. (2003) Intraventricular conduction defects in patients with congestive heart failure: left but not right bundle branch block is an independent predictor of prognosis. A report from the Italian Network on Congestive Heart Failure (IN-CHF database). Ital Heart J 4(9): 607-613.
- Tereshchenko LG, Henrikson CA, Stempniewicz P, Han L, Berger RD (2011) Antiarrhythmic effect of reverse electrical remodeling associated with cardiac resynchronization therapy. Pacing Clin Electrophysiol 34(3): 357-364.
- Malik M, Batchvarov VN (2000) Measurement, interpretation and clinical potential of QT dispersion. J Am Coll Cardiol 36(6): 1749-1766.
- Yan GX, Rials SJ, Wu Y, Liu T, Xu X, et al. (2001) Ventricular hypertrophy amplifies transmural repolarization dispersion and induces early after depolarization. Am J Physiol Heart Circ Physiol 281(5): H1968-H1975.
- Antzelevitch C, Fish J (2001) Electrical heterogeneity within the ventricular wall. Basic Res Cardiol 96: 517-527.
- Panikkath R, Reinier K, Uy-Evanado A, Teodorescu C, Hattenhauer J, et al. (2011) Prolonged Tpeak-to-tend interval on the resting ECG is associated with increased risk of sudden cardiac death. Circ Arrhythm Electrophysiol 4(4): 441-447.
- Haarmark C, Graff C, Andersen MP, Hardahl T, Struijk JJ, et al. (2010) Reference values of electrocardiogram repolarization variables in a healthy population. J Electrocardiol 43(1): 31-39.
- Porthan K, Viitasalo M, Toivonen L, Havulinna AS, Jula A, et al. (2013) Predictive value of electrocardiographic T-wave morphology parameters and T-wave peak to T-wave end interval for sudden cardiac death in the general population. Circ Arrhythm Electrophysiol 6: 690- 696.
- Ypenburg C, van Bommel RJ, Borleffs CJW, Bleeker GB, Boersma E, et al. (2009) Long-term prognosis after cardiac resynchronization therapy is related to the extent of left ventricular reverse remodeling at midterm follow-up. J Am Coll Cardiol 53(6): 483-490.
- Chakir K, Daya SK, Tunin RS, Helm RH, Byrne MJ, et al. (2008) Reversal of global apoptosis and regional stress kinase activation by cardiac resynchronization. Circulation 117(11): 1369-1377.






























