Recurrent Pregnancy loss in Consanguineous family with two different variant identifications by Couple carrier screening
Priyanka Vishwakarma1*, Ashish Dubey1, Shashank Upadhyay2, Amit Joshi2, Deepika Kalo1 and Vishal Kumar Mishra3
1Department of Clinical Genomics, Redcliffe Lifetech Private Limited, Noida, India
2Department of Biotechnology, Invertis University, Bareilly, India
3Department of Bioinformatics, Redcliffe Lifetech Private Limited, Noida, India
Submission: February 14, 2022; Published: March 08, 2022
*Corresponding author: Priyanka Vishwakarma, Department of Clinical Genomics, Redcliffe Lifetech Private Limited, India
How to cite this article: Priyanka V, Ashish D, Shashank U, Amit J, Deepika K, et al. Recurrent Pregnancy loss in Consanguineous family with two different variant identifications by Couple carrier screening. J Gynecol Women’s Health 2022: 23(2): 556106. DOI: 10.19080/JGWH.2022.23.556106
Abstract
We present a rare case of recurrent pregnancy loss associated with autosomal recessive mutation identified by Clinical Exome sequencing (Couple carrier testing Method). The Consanguineous Couple were facing recurrent pregnancy loss before 20 weeks of gestation since last 7 years of marriage. This type of pregnancy loss occurs in approximately 5% of reproductive-aged women, her hormone level, ultrasound, and the biochemical test report were normal. We report two autosomal recessive mutations in two different genes responsible for fetus growth during pregnancy identified in couple and this mutation has been confirmed by Sanger validation method. Study showed the utility of Clinical Exome sequencing method in couple carrier screening to take proper decision in future family planning. Ultimately, the consanguineous marriages are really stigma in our society as a mother of rare disease and responsible for the pregnancy losses as well.
Keywords: Recurrent Pregnancy loss; Abortions; Mutation; Carrier Screening
Abbreviations: RPL: Recurrent Pregnancy Loss; ACOG: American College of Obstetrics and Gynaecology; ACMG: American College of Medical Genetics; SMA: Spinal Muscular Atrophy; ASRM: American Society for Reproductive Medicine; CMA: Chromosomal Microarray; PCS: Preconception Carrier Screening; CF: Cystic Fibrosis; FXS: Fragile X Syndrome; ECS: Expanded Carrier Screening; NGS: Next-Generation Sequencing; PKD: Polycystic kidney disease
Introduction
Spontaneous pregnancy loss is the very common problem of pregnancy. Almost 70% of human conceptions fail to achieve the viability, with almost 50% of all the pregnancies ending in the miscarriage before the clinical acknowledgement of a missed period or the presence of embryonal heart activity [1,2]. Recurrent pregnancy loss (RPL) is less common, taking place in about one out of 100 pregnant women [3]. it has been over all defined as the three or more consecutive pregnancy losses before 20 weeks of the gestational age [2].
An estimated 1% of the couples attempting pregnancy suffer three or more consecutive losses, and as many as 5% have two or more consecutive losses [4]. Causes of RPL can be categorized as genetic abnormalities, hormonal and metabolic disorders, uterine anatomical aberrations, infectious causes, autoimmune disorders, thrombophilic disorders, autoimmune causes, and idiopathic. This latter group accounts for over 50% of the cases [5].
Approximately 50% of the first trimester miscarriages are due to the chromosomal abnormalities in the foetus. Till date, the Trisomies are the most detected abnormalities it is reported approx. 61.2%, followed by triploidies approximately 12.4% cases, monosomy X (10.5%), tetra ploidies (9.2%) and the structural chromosomal abnormalities (4.7%). Most aneuploidies are lethal (Death causing) and the viable trisomies are constrained to only a few human chromosomes.
The most common human trisomy is the chromosome 21 (Down Syndrome). Humans are much abler to tolerate the extra sex chromosomes than extra autosomes. After Down Syndrome the most common human aneuploidy is Klinefelter’s syndrome (47, XXY). On the other hand, cells seem to be particularly sensitive to losing chromosomes, since the only viable human monosomy involves the X chromosome (Turner’s syndrome).
Most often the chromosomal rearrangements in either carrier are a major clinically recognized cause of the miscarriage and these studies are published in a different journal have shown the prevalence of the chromosomal incongruities that varies from 4% to 8% of the couples who are affected by at least two or three pregnancy damages [6-8] Recent recommendations supporting clinical intervention after only two consecutive spontaneous abortions when other features of pregnancy loss are present define a higher prevalence of one in 100 women.
These additional features include detectible fetal heart activity preloss; normal fetal chromosomal content; advanced maternal age; or couple subfertility (Practice Committee of the American Society for Reproductive Medicine 2008a) [9]. Uterine structural abnormalities, endocrinal abnormalities, infections, immunologic factors, metabolic or hormonal disorders, environmental factors, sperm quality, and maternal and paternal age have each been linked to RPL.
The standard RPL estimation presently incorporates the testing for the chromosomal translocations in each of the parent as well as the several maternal testing for endocrine (thyroid), autoimmune (lupus anticoagulant and antiphospholipid antibodies), anatomic (endometrial or uterine abnormalities), and, in some cases, single gene disorders (such as inherited thrombophilias) [10,11].
Despite the number of proposed etiologies, parental chromosomal abnormalities and complications resulting from the antiphospholipid antibody syndrome continue to be the only undisputed causes of RPL. It’s reported in several literatures, that RPL is remains unexplained in 45% to 50% of patients [12]. In most of the cases, there is a poor prognosis that is far from bleak; researchers have shown that the overall possibility of live birth after RPL is 70% –75%, even in women with advanced maternal age [13, 14].
To the cause of these losses, Carrier screening programs were announced in the year 1970s to offer individuals for the opportunity to learn the likelihood that they could pass on an autosomal or X-linked condition to their offspring. Firstly, the carrier screening programs were used only with the ethnic groups who had relatively high incidence of certain conditions, such as ancestry-based screening for Tay–Sachs’s disease in Ashkenazi Jewish communities and βthalassemia in Mediterranean populations [15,16].
The carrier screening testing in the prenatal or at the timing of preconception is suggested for a variety of the conditions based upon the ethnic background and family history. Certain autosomal recessive disease conditions are the more prevalent and reported in the individuals with the specific ancestry or specific to the certain population with their percentage of risk levels. Thus, the couples of the certain populations are at the increased risk for having the offspring with one of such type of conditions. Some of these conditions may be lethal in childhood or are related with significant morbidity.
For Cystic fibrosis the carrier screening is acclaimed by the American College of Obstetrics and Gynaecology (ACOG) for individuals at the stage of preconception and the prenatal periods regardless of ethnic background or the family history. ACOG’s current recommendations indicates that the complete sequencing of the CFTR gene is not appropriate for the routine carrier screening, but carrier screening panels should include at least minimum of 23 most common mutations (ACOG 2017) [17].
It has been recommended by the American College of Medical Genetics (ACMG) and ACOG for the prenatal screening of spinal muscular atrophy (SMA) regardless of family history. Fragile X carrier screening is suggested for women with a family history of fragile X-related disorders, unexplained mental retardation or developmental delay, autism, or premature ovarian insufficiency [18]. Currently Fragile X carrier screening in the general population is not routinely recommended. Individuals of Ashkenazi Jewish descent have an increased risk to have a child with certain autosomal recessive conditions.
The American College of Medical Genetics (ACMG) recommends for the carrier screening for cystic fibrosis, Canavan disease, familial dysautonomia, Tay-Sachs’s disease, Fanconi anemia (Group C), Niemann-Pick (Type A), Bloom syndrome, mucolipidosis IV, and Gaucher disease for all Ashkenazi Jews who are pregnant or considering pregnancy. These disorders all have significant health impact on an affected infant.
RPL testing the American College of Obstetricians and Gynaecologists (ACOG) and the American Society for Reproductive Medicine (ASRM) both are suggested for the chromosomal analysis via karyotyping when a couple has a history of RPL. Karyotype analysis can be performed on either the products of conception or on both parents when a history of RPL is identified. ACMG stated chromosomal microarray (CMA) should not be used to evaluate the parents with the history of RPL, as this technology cannot detect the stable chromosomal rearrangements.
Developing evidence shows that the several advantages of the increasing clinical sensitivity to the Mendelian recessive diseases in the genetic screening of the approaching parents (Preconception carrier screening, PCS). Notably, populationbased incorporation of parallel screening for cystic fibrosis [CF], fragile X syndrome [FXS], and spinal muscular atrophy [SMA] in routine preconception and early pregnancy programs results in a combined affected pregnancy risk comparable to the risk for Down syndrome [19].
Advancement in the sequencing technology and decreases in the cost [20] have made the expanded carrier screening (ECS) reasonable and inexpensive. In 2011, after 14 years of cumulative experience in gene-by-gene carrier screening, screening tests were first expanded to simultaneously test for 448 Mendelian recessive diseases using next-generation sequencing (NGS) technology [21]. Subsequently, ECS has been implemented in the several populations, and the power of the NGS and expanded panels increases detection rates compared with traditional tests [22–25]. Expanded carrier screening does influence reproductive decisions for a high percentage of at-risk couples.
Current documents of guidance do not specify that which conditions should be involved on an expanded panel, but most of them recommended at least some specific conditions such as CF and spinal muscular atrophy [26-28]. There is also consensus among the professional societies that expanded carrier screening panels should focus on the childhood-onset situations that are likely to have a significant impact on the child’s quality of life [29- 33].
In addition to age of onset and clinical impact, most guidance documents also include criteria related to the scope of the condition (including frequency of the gene and penetrance of the phenotype) and the extent to which parents and/or providers can act in response to a positive finding. However, professional societies vary in terms of the specificity of their lists of considerations and/ or criteria, as well as the details of their guidance.
Case Report
Genomic DNA is extracted from the blood samples of the couple presented with the history of recurrent pregnancy loss. who presented for genetic counselling because of three recurrent miscarriages, and they have been married since 7 years. It is the first study in our laboratory. Informed consent was obtained from couple. For NGS, patient DNA corresponding to exonic regions is captured using Agilent targeted Exome hybridization probes. Captured DNA is sequenced by using the Thermofisher’s semiconductor sequencing platform Ion-S5 using 200 bp reads. The following quality control metrics are generally achieved in >97% of target bases are covered at >20x, mean coverage of target bases ~100x.
Data analysis and the variant interpretation has been completed by the grouping of torrent suite software and our internal bioinformatics pipeline. Variants are filtered and interpreted by using the curated databases such as Clinvar, OMIM, dbSNP etc. and common, benign, and low-quality variants are filtered from analysis. All differences from the reference sequences (sequence variants) are assigned to one of five interpretation types (Pathogenic, Likely Pathogenic, Variant of Uncertain Significance, Likely Benign and Benign) as per ACMG Guidelines [34].
All sequence variants in apposite gene regions will be detected and interpreted, but only Pathogenic and Likely Pathogenic variants will be included in the test report. Rare and undocumented synonymous variants are nearly always classified as likely benign if there is no indication that they alter protein sequence or disrupt splicing. Likely benign and benign variants are not included for any sections in report.
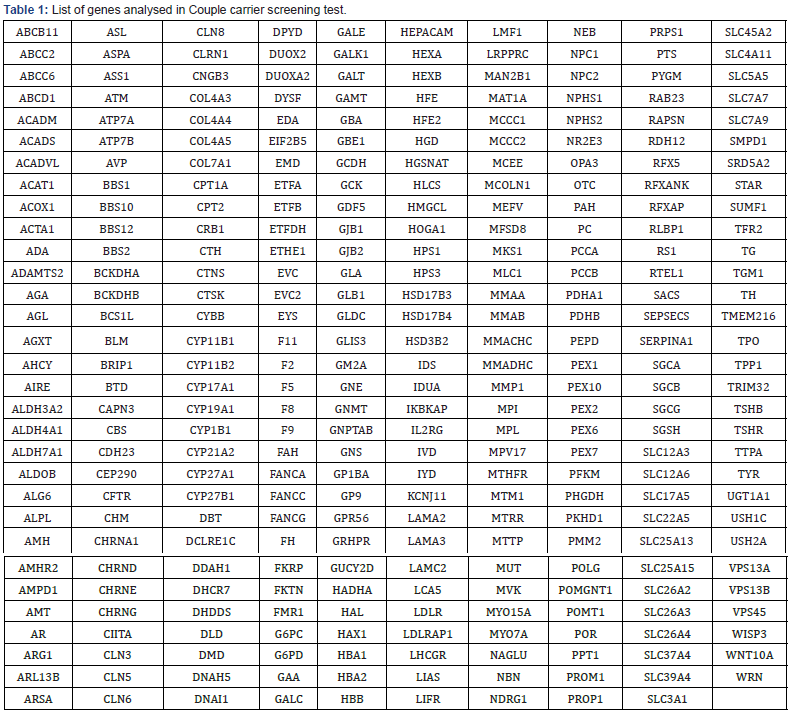
In this study, we tested a panel of over ~300 genes that are associated with around 400 disorders inherited in an autosomal recessive (some X-linked recessive) manner and are mostly very severe and are childhood onset diseases (Table 1). Carrier screening is envisioned for an individual at a reproductive age as a preconception or the prenatal screening to determine if he/she carries one or more mutations for the diseases.
These mutations were designated based on the current American College of Medical Genetics (ACMG) and American College of Obstetrics and Gynaecology (ACOG) commendations, as well as a thorough review of scientific literature and the assessment of their clinical utility. This test is not intended for diagnostic testing of children suspected of having any of the diseases in the panel. Rare false negatives may occur in the setting of bone marrow transplantation, blood transfusion, and genetic variants such as other point mutations and deletions.
The studied couple had the previous history of three recurring abortions with the missing heartbeat in fetus. These couple married for 7 years in the same family (Consanguineous marriage) (Figure 1a). Pedigree of the family showed the 1st degree of consanguinity. After enrollment of this couple, we have performed several hormonal and biochemical tests. Result of the biochemical test has been mentioned in Figure 1b.
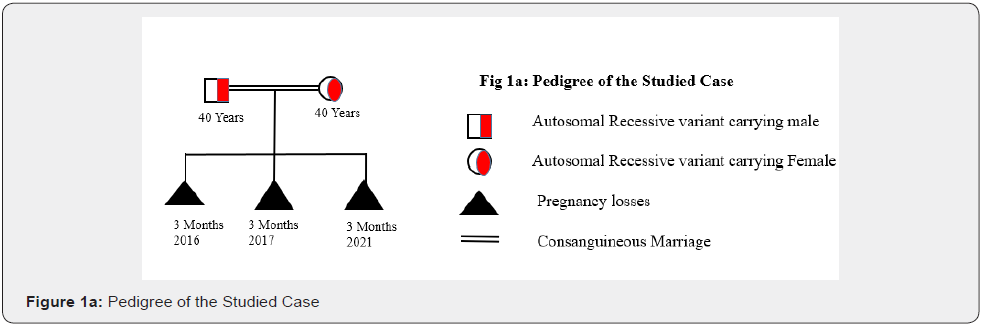
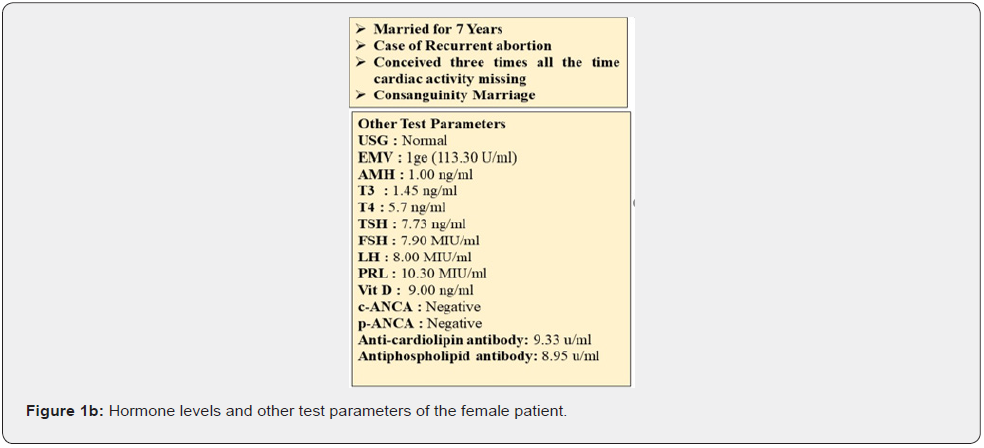
All the test reports were normal, and the ultrasound report was also normal. But all the time this couple faced the pregnancy losses at time of first trimester (3rd month) (Figure 1a). After getting done all the tests, we have enrolled this couple for the couple carrier screening test to know the cause of pregnancy loss.
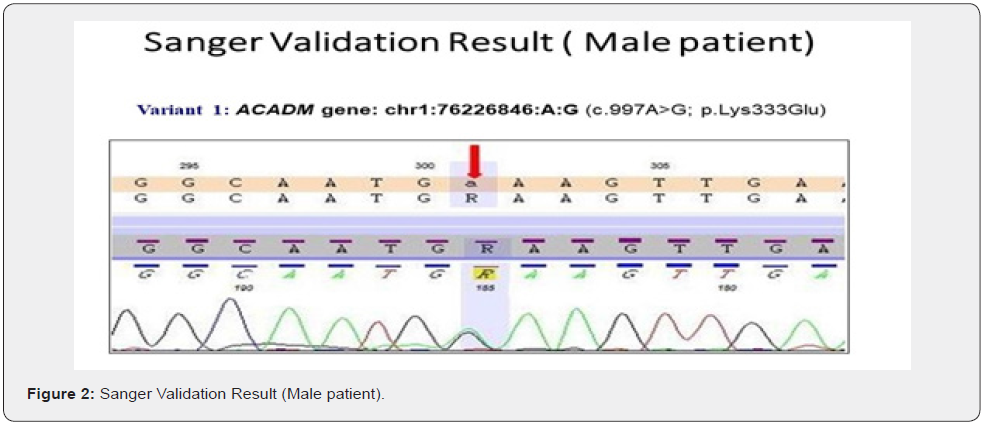
The genes we included in this couple carrier screening test is mentioned in the Table 1. The result of this test indicated this couple is carrier for the two different gene in the two different variants (Table 2&3). The couple found to be carrier of variant c.997A>G; p. Lys333Glu (ACADM gene) and c.732G>C; p. Trp244Cys (PKHD1 gene) (Table 3). All the variant details of identified mutation in couple is mentioned in the table 3.


Both the patients (Husband and Wife) are the carriers of same condition which follow autosomal recessive mode. Combining these results and keeping in view the clinical history, clinician correlated the findings. Genetic counselling has been given to the couple to discuss the potential clinical or reproductive implications of this carrier screening result.
To validate the couple carrier screening results, we have also done Sanger Sequencing (Figure 1 c, d, e, f).
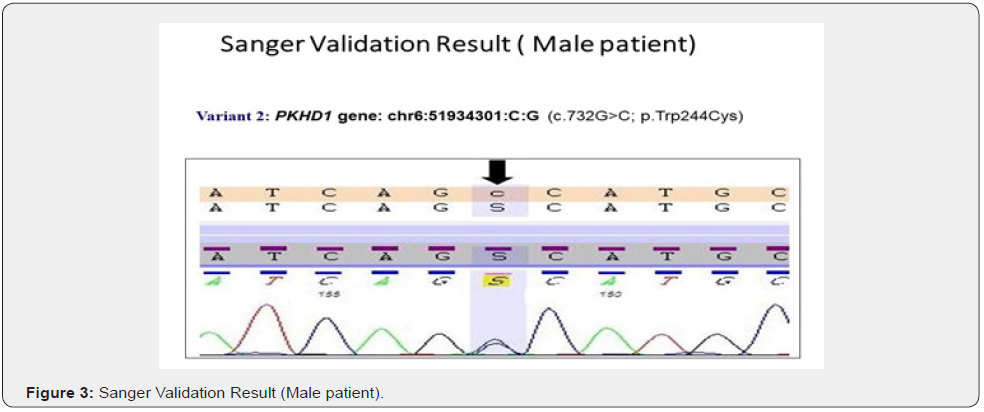
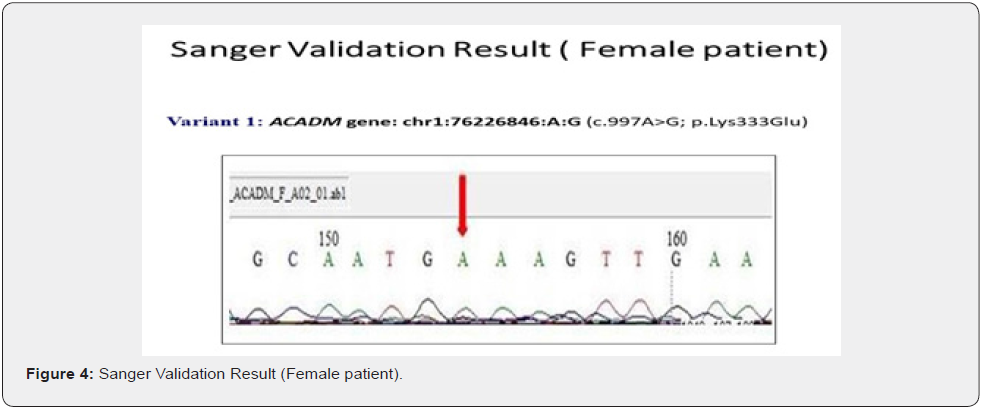
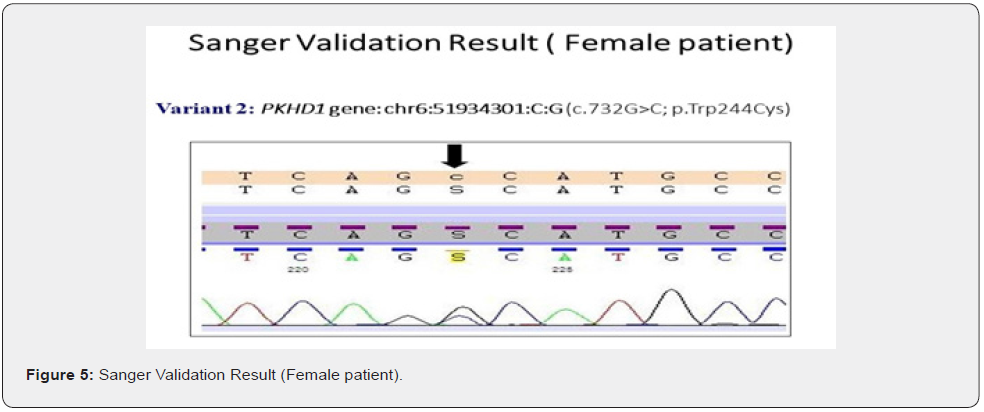
Genetic testing is based upon the information, developments and testing techniques that are known today. Forthcoming research may reveal the changes in the interpretation of previously obtained genetic testing results. Certain genes may not be covered completely, and few mutations may be missed.
We sequence coding exons for each given transcript, plus ~10 bp of flanking non -coding DNA for each exon. Unless specifically indicated, test reports contain no information on about other portions of the gene, such as regulatory domains, deep intronic regions, uncharacterized alternative exons, chromosomal rearrangements, repeat expansions, epigenetic effects, and mitochondrial genome variants. Also, this analysis cannot detect single and multi-exon deletions and duplications.
A negative finding does not rule out the genetic diagnosis. These results should be used in the context of the available clinical findings and should not be used as the sole basis for treatment. As with all medical laboratories testing, there is a small chance that the laboratory could report inaccurate information. For example, the laboratory could report that a given genotype is present when in fact it is not. Any kind of laboratory error may lead to incorrect decisions regarding medical treatment and/or diet and fitness recommendations.
Discussion
Clinical Exome sequencing has transformed the molecular diagnosis of postnatal genetic diseases, but so far it has been used less often to study the reproductive related disorders. Here we provided an overview and the outcomes of the genomic sequencing for detecting the causes of RPL in a couple who suffering from recurrent pregnancy losses. This study includes couple carrier screening by clinical exome sequencing to look for the pathogenic sequence changes in the whole exome or in a preselected list of genes measured to be very important for the early embryonic development and the maintenance of pregnancy.
We developed an approach to diagnose rare autosomal recessive lethal disorders in a consanguineous couple with a history of multiple affected fetuses. The aim was to obtain a molecular genetic diagnosis and enable prenatal testing in the future pregnancies. The result showed that the couple detected with the carrier status of two variants in two different genes. These are ACADM and PKHD1 gene’s variants causing a severe form of fetal Acyl-CoA dehydrogenase, medium chain, deficiency, and Polycystic kidney disease 4, with or without hepatic disease respectively.
These two genes (ACADM & PKDH1) different variants studies have already been reported in the different populations and ethnic groups [35-39]. For the gene ACADM the variants have been reported in European, Caucasian and the Saudi Arabian population with 80% to 95% detection rate. And the carrier frequency rate of this gene is 1 out of 50 cases (Table 2). The ACADM gene delivers the directions for the making an enzyme called medium-chain Acyl-CoA dehydrogenase (MCAD). This enzyme’s function is inside the mitochondria (energy-producing center in cells). MCAD is very important for the fatty acid oxidation, which is the multistep process that breaks down (metabolizes) fats and converts them into the energy.
This MCAD is mandatory to metabolize a group of fats named the medium-chain fatty acids. These fatty acids are found in foods and body fat and are produced when larger fatty acids are metabolized. Fatty acids are a major source of energy for the heart and muscles. During periods without food (fasting), fatty acids are also an important energy source for the liver and other tissues.
For the gene PKDH1 the variant has been reported mostly in the Caucasian and finish population with 20% to 75% detection rate. And the carrier frequency rate of this gene is 1 out of 71 case (Table 2). Polycystic kidney disease (PKHD1 related) causes cysts (fluid-filled sacs) to develop on the kidneys and restrict their ability to filter waste from the blood. PKD causes enlarged kidneys, which can lead to kidney failure. Cysts may also develop in other organs, including the liver, and other symptoms include underdeveloped lungs, heart valve problems and high blood pressure.
Symptoms are usually present from birth, though some people are more mildly affected than others. Treatment through dialysis and kidney transplant can reduce the seriousness of the condition. We conclude that these variants are highly responsible for the disease causing. Diagnosing the lethal foetal disorders has previously been very difficult because of the presence of the large number of potential genes, the phenotypic variability associated with many known genetic causes and the challenges of defining phenotype and pathology in a mid-gestation foetus.
Sequencing of the parental samples overcomes issues of limited quality or the quantity of foetal samples. A genetic diagnosis is must to confirms or identify the risk for future offspring and to get permission in the early prenatal diagnosis or preimplantation genetic diagnosis in future pregnancies.
This in turn reduces the anxiety associated with the waiting until mid-pregnancy for an ultrasound diagnosis and avoids the added suffering of a late cessation of the pregnancy period. This strategy is also valid to those disorders not detectable by the ultrasound diagnosis where late foetal demise or a neonatal death could not otherwise be predicted.
Conclusion
RPL is a condition which has both the psychological and the economical adverse effects on both for the couples and scientific experts dealing with these patients. Emphasizing the real reason behind these cases will be beneficial for both patients and the experts. This is very important to emphasize that the consanguineous marriages are so much responsible for these types of genetic disorders.
Acknowledgment
We are very grateful for patient’s participation in this study and the clinician who has refereed this case at our company.
References
- Edmonds DK, Lindsay KS, Miller JF, Williamson E, Wood, PJ, et al. (1982) Early embryonic mortality in women. Fertil Steril 38(4): 447–453.
- Wilcox AJ, Weinberg CR, O’Connor JF, Baird DD, Schlatterer JP, et al. (1988) Incidence of early loss of pregnancy. N Engl J Med 319(4): 189 -194.
- Alberman E (1988) The epidemiology of repeated abortion. In Early pregnancy loss: Mechanisms and treatment, (ed. Beard RW and Sharp F) Springer-Verlag, USA, pp. 9 -17.
- Sierra S, Stephenson MD (2006) Genetics of recurrent pregnancy loss. Semin. Reprod. Med 24(1): 17–24.
- Stephenson MD, Salim D (1996) Frequency of factors associated with habitual abortion in 197 couples. Fertil Steril 66(1): 24-29.
- Niroumanesh S, Mehdipour P, Farajpour A, Darvish S (2011) A cytogenetic study of couples with repeated spontaneous abortions. Ann. Saudi. Med 31(1): 77‑79.
- Faeza AMED (2011) Chromosomal abnormalities as a cause of recurrent abortions in Egypt. Indian. J. Hum. Genet 17(2): 82‑84.
- Tunç E, Tanrıverdi N, Demirhan O, Süleymanova D, Çetinel N, et al. (2016) Chromosomal analyses of 1510 couples who have experienced recurrent spontaneous abortions. Reprod. Biomed Online 32(4): 414‑419.
- Practice Committee of the American Society for Reproductive Medicine (2008) Fertil Steril 89(6): 1603.
- Sierra S, Stephenson MD (2006) Genetics of recurrent pregnancy loss. Semin. Reprod. Med 24(1): 17-24.
- Practice Committee of the American Society for Reproductive Medicine (2012) Evaluation and treatment of recurrent pregnancy loss: A committee opinion. Fertil. Steril 98(5): 1103–1111.
- Stephenson M, Kutteh W (2007) Evaluation and management of recurrent early pregnancy loss. Clin. Obstet. Gynecol 50(1): 132 –145.
- Clifford K, Rai R, Regan L (1997) Future pregnancy outcome in unexplained recurrent first trimester miscarriage. Hum. Reprod 12(2): 387 –389.
- Brigham SA, Conlon C, Farquharson RG (1999) A longitudinal study of pregnancy outcome following idiopathic recurrent miscarriage. Hum. Reprod 14(11): 2868–2871.
- Kaback MM (2000) Population-based genetic screening for reproductive counseling: the Tay-Sachs disease model. Eur. J. Pediatr 159 Suppl 3: S192–S195.
- Cao A, Saba L, Galanello R, Rosatelli MC (1997) Molecular diagnosis and carrier screening for beta thalassemia. JAMA 278(15): 1273–1277.
- Elliott AM, Radecki J, Moghis B, Li X, Kammesheidt A, et al. (2012) Rapid detection of the ACMG/ACOG-recommended 23 CFTR disease-causing mutations using ion torrent semiconductor sequencing. J Biomol Tech 23(1): 24-30.
- (2010) ACOG Committee Opinion No. 469: Carrier screening for fragile X syndrome. Gynecol 116(4): 1008-1010.
- Coulam CB (1991) Epidemiology of recurrent spontaneous abortion. Am J Reprod Immunol 26(1): 23-27.
- National Human Genome Research Institute. The cost of sequencing a human genome, Vol 2016.
- Callum JB, Darrell LD, Neil AM, Shannon LH, Elena EG, et al. (2011) Carrier testing for severe childhood recessive diseases by next-generation sequencing. Sci. Transl. Med 3(65): 65ra4.
- Kyle AB, Dale M, Kenny KW, Gregory JH, Kambiz K, et al. (2018) Systematic design and comparison of expanded carrier screening panels. Genet Med 20(1): 55-63.
- Brett B, Susan H, Kinnari U, Philip M, Shai Carmi, et al. (2016) Expanded genetic screening panel for the Ashkenazi Jewish population. Genet Med 18(5): 522–8.
- Julio M, Asan, Yuting Y, Trinidad A, Beatriz RI, et al. (2015) Comprehensive carrier genetic test using next-generation deoxyribonucleic acid sequencing in infertile couples wishing to conceive through assisted reproductive technology. Fertil Steril 104(5): 1286-1293.
- Haque IS, Lazarin GA, Kang HP, Evans EA, Goldberg JD, et al. (2016) Modeled fetal risk of genetic diseases identified by expanded carrier screening. JAMA 316(7): 734-742.
- Archibald AD, Smith MJ, Burgess T, Scarff KL, Elliott J, et al. (2018) Reproductive genetic carrier screening for cystic fibrosis, fragile X syndrome, and spinal muscular atrophy in Australia: outcomes of 12,000 tests. Genet Med 20(5): 513-523.
- Prior TW (2008) Carrier screening for spinal muscular atrophy. Genet Med 10(11): 840-842.
- The Royal Australian and New Zealand College of Obstetricians and Gynecologists (RANZCOG) (2015) Prenatal screening and diagnosis of chromosomal and genetic conditions in the fetus in pregnancy.
- Janice GE, Gerald F, James G, Anthony RG, Mary EN, et al. (2015) Expanded carrier screening in reproductive medicine—points to consider: a joint statement of the American College of Medical Genetics and Genomics, American College of Obstetricians and Gynecologists, National Society of Genetic Counselors, Perinatal Quality Foundation, and Society for Maternal Fetal Medicine. Obstet. Gynecol 125(3): 653-662.
- (2017) Committee on Genetics. Committee opinion no. 690: carrier screening in the age of genomic medicine. Obstet Gynecol 129(3): e35-e40.
- Lidewij H, Pascal B, Davit C, Martina CC, Carla GVE, et al. (2017) Responsible implementation of expanded carrier screening. Eur J Hum Genet 25(11): 1291.
- Douglas WR, Isabelle DB, Christine MA, Richard NB, Carla C, et al. (2016) Joint SOGC-CCMG opinion for reproductive genetic carrier screening: an update for all Canadian providers of maternity and reproductive healthcare in the era of direct-to consumer testing. J Obstet Gynaecol Can 38(8): 742-762.e3.
- Superior Health Council (SHC) Belgium (2017) Advisory report of the Superior Health Council no. 9240-expanded carrier screening in a reproductive context. Towards a responsible implementation in the healthcare system.
- Richards S, Aziz N, Bale S, Bick D, Das S, et al. (2015) On behalf of the ACMG Laboratory Quality Assurance Committee, Standards and Guidelines for the Interpretation of Sequence Variants: A Joint Consensus Recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genetics in Medicine: Official Journal of the American College Of Medical Genetics 17(5): 405- 424.
- Obeidova L, Seeman T, Elisakova V, Reiterova J, Puchmajerova A, et al. (2015) Molecular genetic analysis of PKHD1 by next-generation sequencing in Czech families with autosomal recessive polycystic kidney disease. BMC Med Genet 16: 116.
- Jiwon J, Hun GS, Yoo MK, Young MH, Ji KP, et al. (2020) Fatal outcome of autosomal recessive polycystic kidney disease in neonates with recessive PKHD1 mutations. Medicine (Baltimore) 99(19): e20113.
- Pankaj T, Paul S, Aleksandar R (2014) Novel Mutation in the PKHD1Gene Diagnosed Prenatally in a Fetus with Autosomal Recessive Polycystic Kidney Disease. Case Reports In Genetics 2014: 6
- Jing C, Na M, Xiaomeng Z, Wen L, Qianjun Z, et al. (2019) A rare deep intronic mutation of PKHD1 gene, c.8798-459 C > A, causes autosomal recessive polycystic kidney disease by pseudoexon activation. J Hum Genet 64(3): 207-214.
- Sarah CG, Wehrle A, Villavicencio LP, Lausch E, Vetter B, et al. (2015) Medium-chain acyl-CoA dehydrogenase deficiency associated with a novel splice mutation in the ACADM gene missed by newborn screening. BMC Medical Genetics 16: 56.






























