CKD Uraemic Environment does not Influence Adiponectin Secretion- A “Bed-To-Bench” Approach
IJoachim Beige1*, Susanne Winkelhog2 and Stefan Engeli3
1Division of Nephrology and KfH Renal Unit, Hospital St. Georg, Germany
2Faculty of Medicine, Martin-Luther-University, Germany
3Institute of Clinical Pharmacology, Hannover Medical School, Germany
Submission: February 23, 2017; Published: March 31, 2017
*Corresponding author: Joachim Beige, MD, Division of Nephrology, Klinikum St. Georg gGmbH, Delitzscher Str. 141, 04129 Leipzig, Germany,Email: joachim.beige@kfh-dialyse.de
How to cite this article: Joachim B, Susanne W, Stefan E.CKD Uraemic Environment does not Influence Adiponectin Secretion- A “Bed-To-Bench” Approach. J Endocrinol Thyroid Res. 2017; 1(3): 555564. DOI:10.19080/JETR.2017.01.555564
Abstract
Adiponectin (ADPN) is an adipocyte-derived protein carrying anti-atherogenic properties in the general population. Controversially, ADPN serum concentrations are positively associated with the magnitude of chronic kidney disease (CKD), which is the strongest cardiovascular risk factor per se. This piece of reverse epidemiology raises the question, if ADPN is stimulated by uremia itself or is simply a bystander of CKD. To enlighten this question on the cellular level, we performed co-stimulatory experiments in human adipocytes using insulin- an established ADPN stimulating agent- and compound uremic haemofiltrate from patients with CKD stage 5. Haemofiltrate was employed as a model fluid because the more comprehensive approach of using uremic serum was methodologically impossible due to the ADPN content of serum.
Differentiated adipocytes were treated with compound uraemic haemofiltrate from patients with CKD stage 5 or PBS as control. Surprisingly, we did not detect any additional stimulatory effect of increasing haemofiltrate concentrations on adiponectin secretion of human adipocytes after 24 and 48 hours of incubation (p=0.67 or 0.07 resp.).
Therefore, this in vitro experiment preliminary precludes a pathophysiological effect of water-soluble uremic compounds on ADPN cellular secretion. Further experiments with varying amounts of insulin and other ADPN stimulators are needed to confirm such preclusion.
Introduction
Adiponectin (ADPN) is an adipocyte-derived protein [1] with potential antiatherogenic properties and exclusively secreted by adipocytes. In chronic kidney disease (CKD), ADPN serum levels are increased [2-7] for unknown reasons although accumulation due to abolished clearance [8-10] or modified metabolic pathways [4] has been hypothesized but not finally proved. The ADPN increase in CKD is of certain biological interest, because in the general population ADPN serum levels are inversely associated with cardiovascular risk [11-15]. The underlying vascular-protective effects are mounted on several mechanisms like insulin sensitizing [16-19], maintaining endovascular homeostasis [20] and anti-inflammatory properties [21,22]. In opposite, patients with chronic kidney disease (CKD) are subjected to an increased cardiovascular risk [23,24] in general and by even higher extent, if they exhibit higher ADPN serum values [3,6,25,26]. Therefore, the finding of increased ADPN in CKD and increased mortality risk along with higher ADPN can be regarded as a paradox or a further finding of reverse epidemiology [5]. It must be challenged, whether in CKD ADPN plays the same protective role as it is supposed to do in the general population.
Osteoclast activation in CKD may be regarded as one of the candidate mechanisms to be differentially effective in CKD vs. normal environment [27]. Because osteoclasts are being activated via NFkB [28] and the Receptor Activator of NFkB Ligand (RANKL) [29], this pathway could be seen as a hypothetical interplay with ADPN regulation. ADPN was shown to stimulate RANKL in vitro and inhibited osteoprotegrin (OPG) in osteoblasts. In opposite, ADPN was found to be positively associated with OPG [30], which is indeed a decoy receptor for NFkB and therefore protects endothelial integrity [31,32]. Hypothetically, the molecular role of ADPN in CKD could be seen inversely to it´s role in other populations, since it´s induction might activate osteoclasts and calcium- phosphate liberation from bones becoming osteopenic. With such regard, the impact of ADPN could be a function of renal disease and the particular milieu in CKD.
As a step to clarify the underlying mechanism we aimed to decipher whether ADPN secretion by adipocytes might be influenced by uremia itself, initially by deployment of a comprehensive variety of uremic compounds as stimulating agent for ADPN secretion of adipocytes. Because serum of CKD patients contains such comprehensive assortment, but also ADPN in a large quantity, it is technically difficult or not possible to separate detection results between serum ADPN and ADPN secreted by adipocytes after stimulation. The present study examined insulin-stimulated nearly mature adipocytes incubated with uremic, but ADPN-free haemofiltrate from endstage, dialysis-necessitating CKD patients to analyse ADPN secretion under uremic conditions.
Methods
Cell culture
To test ADPN secretion, preadipocytes isolated from a patient with Simpson-Golabi-Behmel syndrome (SGBS) were studied. These immortal cells can easily be differentiated into nearly mature adipocytes by insulin and are specifically useful to study adipocyte differentiation, metabolism, and secretory properties.
Cells were seeded in culture flasks by a density of 4000/cm2 and cultivated in Dulbecco’s Modified Eagle’s Medium (DMEM) (Invitrogen, Karlsruhe, Germany). When cell confluency reached 80%, cells were washed threefold with phosphate-buffersaline (PBS, Invitrogen) and insulin-induced 3 days with DMEM enriched by 20μM insulin, 10% fetal calf serum, 300mm Biotin (Sigma-Aldrich, Seelze, Germany), 150mm Panthothenat (Sigma- Aldrich, Seelze, Germany), 100U/ml Penicillin (Sigma-Aldrich, Seelze, Germany) and 100mg/ml Streptomycin (Sigma-Aldrich, Seelze, Germany), in order to induce adipogenic differentiation. After 9 days, differentiation medium (insulin-containing) was replaced by the investigational (insulin-free) culture medium. That medium consisted of DMEM with varying amounts of pooled uremic haemofiltrate (HF) or PBS. To adjust for changes in nutrient concentrations in dilution series, PBS or uraemic solution were added at similar volumes yielding constant nutrient concentration in control and uremia-supplemented dishes at each uraemia concentration. Experiments were performed both for concentration and time dependency.
Visual cell behavior and confluency control was used for viability control. Using such approach, all ADPN experiments were done with a fixed amount of culturing solution but varying amounts of uremic solution or buffer solution serving as an internal control (uremic co-incubation).
Haemofiltrates and patients
CKD patients who exhibite large interdialytic fluid gain can routinely being treated by a sequential dialysatefree, pure haemofiltration (HF) phase during their regular outpatient dialysis sessions to reach better hemodynamic stability [33]. During these initial phases (mostly about 30min) without any dialysate flow (not to be confused with standardhemodiafiltration including dialysate flow), HF fluid from the wasting outlets of dialysis machines was drawn and pooled for further experimental investigations. The volume of HF fluid was between 100 and 500mL during the first 30min of session. It represents ultrafiltrated uremia serum and a non-specified, not diluted mixture of water-soluble small uremic compounds in a small volume and was used as stock solution for subsequent dilution series. Because no individual patient data were collected and no interventional therapy modification was conducted, patient were informed about machine fluid collection, but no formal written consent was sought.
ADPN measurements
Supernatants from cells cultured in medium with uremic augmentation (HF) ore PBS addition were harvested in two experiments. Experiment A employed a composition of the investigational medium containing 30% haemofiltrate together with 70% PBS vs. a control medium of 100% PBS and time points of 8, 24, 36, 48 and 60 hours with threefold ADPN measurement at each time-point. Experiment B used variable uremic haemofiltrate concentrations of 0, 10, 20, 30, 40 and 50% (with the replacing amount of PBS, resp.) at time points 24 and 48 hours with ADPN measurement in triplicate. Every uremic concentration was conducted twice with triplicate ADPN measurement. Total ADPN was measured by sheep alcalinephosphatase based antibody ELISA assay (Biovendor, Heidelberg, Germany, Cat.# RSCHHMWADN096R). The assay was calibrated according the user´s manual by means of a pretested calibration curve. Each data-point displays the mean of 3 measurements and standard deviation.
Statistical analysis
For comparison of correlations between uremic concentration, incubation time and ADPN concentration, linear regression and ANOVA was used. The mean ADPN change per % change of HF proportion is given as 95% confidence interval. For comparison of 24h vs. 48h time-points and uremic augmentation vs. PBS, paired t-test was used.
Results
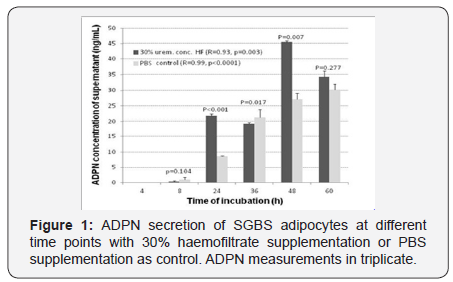
Supernatant total ADPN concentration and time of incubation were highly correlated both with (Pearson´s R 0.92; p=0.003)and without (Pearson´s R 0.99; p<0.0001) 30% HF co-incubation (Figure 1). At time points 24 and 48 hours, the supernatant ADPN concentration was higher in uremic solutions (p<0.001, p=0.007) compared to PBS while the incremental increase of ADPN with augmented uremic environment did not reach correlation with the incremental increase of ADPN over time (p=0.1, Figure 1).
Supernatant total ADPN concentration and concentration of uremic co-incubation fluid were not correlated at 24 hours (Pearson´s R 0.14; p=0.67) and 48 hours (Pearson´s R-0.59; p<0.067) (Figure 2).
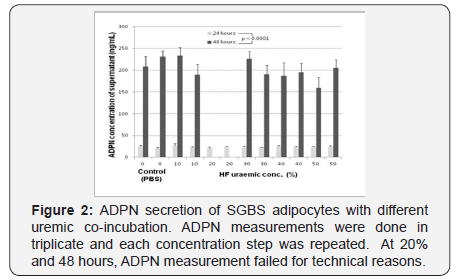
ADPN levels in both non-uremic and serial augmented uremic environment were about 10times higher (95%CI D 162...195; p< 0.0001) after an incubation period of 48 hours compared to 24 hours (p<0.0001).
Discussion
By applying a uraemic environment of small water soluble molecules on secreting adipocytes in an artificial setting, overt and continuing ADPN stimulation was not notable on the background of initial one-time insulin stimulation. The 1.8fold ADPN increase responding to 30% uremic concentration was only present at time-points 24 and 48 hours, was not disproportionate to the ADPN time dependency and could not be replicated with varying concentrations of uremic environment. We applied an assay based on SGBS cells comparable to other in vitro studies with a similar technical approach. The tremendous time course of ADPN secretion may indicate that peak ADPN secretion was not reached at the 24-hours-time-point and our experiments were done in a linear phase of the ADPN timesecretion relationship. Such reasoning points to the assumption, that uremic environment was present during a non-plateau and secreting phase of ADPN production but did not carry major stimulatory effects exceeding insulin effects. These results can be interpreted as a preliminary preclusion of an in-vitro stimulatory effect of uraemia on ADPN secretion.
At the clinical level, other researchers have investigated if accumulation along with diminished renal function might play a role in the paradox of high ADPN in CKD. Studies demonstrated ADPN total and HMW isoforms to be increased in dialysis patients [14], in renal transplantation but also in nephrotic syndrome [4]yielding even higher levels compared to stage 5 CKD. This might suggest that ADPN levels are associated with lipid metabolism but not so much with accumulation in renal failure.
In vitro studies like the present one carry shortcomings when comparisons to clinical situations are drawn. First, by using concentrated haemofiltrate from the first period of conventional dialysis, we were able to draw a mixture of small water-soluble, but not protein-bound and larger molecules because these molecules do pass the dialysis filter membrane by only a minor proportion [34]. Methodological, we tested total ADPN, although distinct biological effects of HMW isoforms have been suggested [35]. However, there is available evidence that total and HMW ADPN are correlated and total ADPN assays have been applied as satisfying surrogates [14]. Of course, biological cofactors like lipid metabolism, inflammation and bone metabolism could not have been simulated. The present results were mounted on the background of initial insulin stimulation to accomplish adipogenic differentiation. By such procedure we aimed to reach comparison with known physiological mechanism (insulin dependency) with the hypothesized uraemia dependency. Because this was not the case, we may conclude that uraemia at least does not play a role as insulinemia does. We cannot draw conclusions regarding smaller increments, e.g. in terms of not insulin-dependent systems.
Therefore our initial investigational findings do only add first reasoning that adipocyte ADPN secretion is not enhanced overtly by uraemia. Further steps, using modified insulin and other costimulating compounds as well as protein-bound compounds seem to be meaningful to widen the perception of cellular ADPN secretion in frame of CKD.
References
- Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF (1995) A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem 270(45): 26746-26749.
- Bakkaloglu SA, Buyan N, Funahashi T, Pasaoglu H, Elhan AH, et al. (2005) Adiponectin levels and atherosclerotic risk factors in pediatric chronic peritoneal dialysis patients. Perit Dial Int 25(4): 357-361.
- Bakkaloglu SA, Soylemezoglu O, Buyan N, Oktar SO, Funahashi T, et al. (2006) Adiponectin levels and arteriosclerotic risk factors in pediatric renal transplant recipients. Pediatr Transplant 10(2): 187-192.
- Bakkaloglu SA, Soylemezoglu O, Buyan N, Funahashi T, Elhan AH, et al. (2005) High serum adiponectin levels during steroid-responsive nephrotic syndrome relapse. Pediatr Nephrol 20(4): 474-477.
- Beige J, Heipmann K, Stumvoll M, Korner A, Kratzsch J (2009) Paradoxical role for adiponectin in chronic renal diseases? An example of reverse epidemiology. Expert Opin TherTargets 13(2): 163-173.
- Zoccali C, Mallamaci F, Tripepi G, Benedetto FA, Cutrupi S, et al. (2002) Adiponectin, metabolic risk factors, and cardiovascular events among patients with end-stage renal disease. J Am Soc Nephrol 13(1): 134- 141.
- Shen YY, Charlesworth JA, Kelly JJ, Loi KW, Peake PW (2007) Upregulation of adiponectin, its isoforms and receptors in end-stage kidney disease. Nephrol Dial Transplant 22(1): 171-178.
- Koshimura J, Fujita H, Narita T, Shimotomai T, Hosoba M, et al. (2004) Urinary adiponectin excretion is increased in patients with overt diabetic nephropathy. Biochem Biophys Res Commun 316(1): 165-169.
- Shimotomai T, Kakei M, Narita T, Koshimura J, Hosoba M, et al. (2005) Enhanced urinary adiponectin excretion in IgA-nephropathy patients with proteinuria. Ren Fail 27(3): 323-328.
- Shen YY, Hughes JT, Charlesworth JA, Kelly JJ, Peake PW (2008) Adiponectin is present in the urine in its native conformation, and specifically reduces the secretion of MCP-1 by proximal tubular cells. Nephrology (Carlton) 13(5): 405-410.
- Engl J, Bobbert T, Ciardi C, Laimer M, Tatarczyk T, et al. (2007) Effects of pronounced weight loss on adiponectin oligomer composition and metabolic parameters. Obesity (Silver Spring) 15(5): 1172-1178.
- Scoditti E, Massaro M, Carluccio MA, Pellegrino M, Wabitsch M, et al. (2015) Additive regulation of adiponectin expression by the mediterranean diet olive oil components oleic Acid and hydroxytyrosol in human adipocytes. PloS One 10(6): e0128218.
- Seino Y, HiroseH, Saito I, Itoh H (2007) High molecular weight multimer form of adiponectin as a useful marker to evaluate insulin resistance and metabolic syndrome in Japanese men. Metabolism 56(11): 1493- 1499.
- Aso Y, Yamamoto R, Wakabayashi S, Uchida T, Takayanagi K, et al. (2006) Comparison of serum high-molecular weight (HMW) adiponectin with total adiponectin concentrations in type 2 diabetic patients with coronary artery disease using a novel enzyme-linked immunosorbent assay to detect HMW adiponectin. Diabetes 55(7): 1954-1960.
- Hara K, Horikoshi M, Yamauchi T, Yago H, Miyazaki O, et al. (2006) Measurement of the high-molecular weight form of adiponectin in plasma is useful for the prediction of insulin resistance and metabolic syndrome. Diabetes Care 29(6): 1357-1362.
- Civitarese AE, Jenkinson CP, Richardson D, Bajaj M, Cusi K, et al. (2004) Adiponectin receptors gene expression and insulin sensitivity in nondiabetic Mexican Americans with or without a family history of Type 2 diabetes. Diabetologia 47(5): 816-820.
- Yamauchi T, Kamon J, Ito Y, Tsuchida A, Yokomizo T, et al. (2003) Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature 423(6941): 762-769.
- Yamauchi T, Kamon J, Waki H, Imai Y, Shimozawa N, et al. (2003) Globular adiponectin protected ob/ob mice from diabetes and ApoEdeficient mice from atherosclerosis. J Biol Chem 278(4): 2461-2468.
- Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, et al. (2002) Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med 8(11): 1288-1295.
- Chen H, Montagnani M, Funahashi T, Shimomura I, Quon MJ (2003) Adiponectin stimulates production of nitric oxide in vascular endothelial cells. J Biol Chem 278(45): 45021-45026.
- Dervisoglu E, Eraldemir C, Kalender B, Kir HM, Caglayan C (2008)Adipocytokines leptin and adiponectin, and measures of malnutritioninflammation in chronic renal failure: is there a relationship? J Ren Nutr 18(4): 332-337.
- Yamamoto K, Kiyohara T, Murayama Y, Kihara S, Okamoto Y, et al. (2005) Production of adiponectin, an anti-inflammatory protein, in mesenteric adipose tissue in Crohn’s disease. Gut 54(6): 789-796.
- Foley RN, Parfrey PS, Sarnak MJ (1998) Epidemiology of cardiovascular disease in chronic renal disease. J Am Soc Nephrol 9(12 Suppl): S16-S23.
- Foley RN, Murray AM, Li S, Herzog CA, McBean AM, et al. (2005) Chronic kidney disease and the risk for cardiovascular disease, renal replacement, and death in the United States Medicare population, 1998 to 1999. J Am Soc Nephrol 16(2): 489-495.
- Iwashima Y, Katsuya T, Ishikawa K, Ouchi N, Ohishi M, et al. (2004) Hypoadiponectinemia is an independent risk factor for hypertension. Hypertension 43(6): 1318-1323.
- Iwashima Y, Horio T, Kumada M, Suzuki Y, Kihara S, et al. (2006) Adiponectin and renal function, and implication as a risk of cardiovascular disease. Am J Cardiol 98(12): 1603-1608.
- Schlieper G, Westenfeld R, Brandenburg V, Ketteler M (2007) Inhibitors of calcification in blood and urine. Semin Dial 20(2): 113-121.
- Tsao TS, Murrey HE, Hug C, Lee DH, Lodish HF (2002) Oligomerization state-dependent activation of NF-kappa B signaling pathway by adipocyte complement-related protein of 30 kDa (Acrp30). J Biol Chem 277(33): 29359-29362.
- Kobayashi Y, Udagawa N, Takahashi N (2009) Action of RANKL and OPG for osteoclastogenesis. Crit Rev Eukaryot Gene Expr 19(1): 61-72.
- Gannage-Yared MH, Fares F, Semaan M, Khalife S, Jambart S (2006) Circulating osteoprotegerin is correlated with lipid profile, insulin sensitivity, adiponectin and sex steroids in an ageing male population. Clin Endocrinol (Oxf) 64(6): 652-658.
- Pritzker LB, Scatena M, Giachelli CM (2004) The role of osteoprotegerin and tumor necrosis factor-related apoptosis-inducing ligand in human microvascular endothelial cell survival. Mol Biol Cell 15(6): 2834-2841.
- Malyankar UM, Scatena M, Suchland KL, Yun TJ, Clark EA, et al. (2000) Osteoprotegerin is an alpha vbeta 3-induced, NF-kappa B-dependent survival factor for endothelial cells. J Biol Chem 275(28): 20959-20962.
- Petras D, Fortunato A, Soffiati G, Brendolan A, Bonello M, et al. (2005) Sequential convective therapies (SCT): a prospective study on feasibility, safety, adequacy and tolerance of on-line hemofiltration and hemodiafiltration in sequence. Int J Artif Organs 28(5): 482-488.
- Eloot S, Schneditz D, Cornelis T, Van Biesen W, Glorieux G, et al. (2016) Protein-Bound Uremic Toxin Profiling as a Tool to Optimize Hemodialysis. PloS One 11(1): e0147159.
- Lara-Castro C, Luo N, Wallace P, Klein RL, Garvey WT (2006) Adiponectin multimeric complexes and the metabolic syndrome trait cluster. Diabetes 55(1): 249-259.






























