Herd-Level Prevalence of Coxiella Burnetii in Bulk Tank Milk and Associated Dairy Farm Characteristics in Southern New England, USA
Meera S Gatlin*
Department of Infectious Disease and Global Health, Cummings School of Veterinary Medicine at Tufts University, Grafton, MA, 01536, USA
Submission: June 06, 2024; Published: June 20, 2024
*Corresponding author: Meera S Gatlin, Department of Infectious Disease and Global Health, Cummings School of Veterinary Medicine at Tufts University, Grafton, MA, 01536, USA
How to cite this article: Meera S Gatlin*. Herd-Level Prevalence of Coxiella Burnetii in Bulk Tank Milk and Associated Dairy Farm Characteristics in Southern New England, USA. Dairy and Vet Sci J. 2024; 16(4): 555946..DOI: 10.19080/JDVS.2024.16.555946
Abstract
Herd-level prevalence of Coxiella burnetii was assessed in 78 dairy cattle operations selling raw milk and milk intended for pasteurization (commercial milk) in Connecticut, Rhode Island, and Massachusetts, using bulk tank milk sampling and PCR analysis. C. burnetii was detected in 40% of operations, including 21% of raw milk farms and 53% of commercial milk farms. Commercial milk dairy farms were 4.3 times more likely to test positive than raw milk dairy farms. However, when stratified by herd size, small raw and commercial dairies were equally likely to test positive for C. burnetii. A survey of management practices showed farms with high somatic cell counts greater than 200,000 cells/mL and regular use of cooling fans increased odds of testing positive for C. burnetii, while use of fore-stripping techniques decreased odds. A survey of raw milk consumption habits showed that 100% of raw dairy producers and 64% of commercial dairy producers consumed raw milk from their farm. Given its zoonotic potential, the high prevalence of C. burnetii in bulk tank milk and raw milk consumption rates on dairy cattle farms indicate it is a significant public health risk in southern New England.
Keywords: public health; cattle; Coxiella burnetiid; Q Fever; bulk tank; milk; raw milk; zoonoses
Abbreviations: CDC: Centers for Disease Control and Prevention; BTM: Bulk Tank Milk; Rt-PCR: Real-Time Polymerase Chain Reaction; IRB: Institutional Review Board; BLV: Bovine Leukosis Virus
Introduction
Raw, or unpasteurized, dairy milk consumption has increased in popularity in the United States due to the perception that pasteurization destroys the nutritional health benefits of milk. The U.S. Centers for Disease Control and Prevention (CDC) [1] reports that nearly 3% of the population drinks raw milk, which amounts to 9 million consumers in the United States today. Raw milk advocates believe it contains higher levels of minerals, vitamins, enzymes, and other natural ingredients [2]. However, federal agencies such as the U.S. Food and Drug Administration [3] argue that raw milk poses severe health risks to consumers, demonstrating the association of raw milk with numerous pathogenic organisms. A study conducted by the CDC characterized U.S. milk-borne disease outbreaks from 1993-2006 and reported that out of 121 cases, over half were caused by raw milk consumption; most of the outbreaks were in one of 30 states that permit the sale of raw milk [1].
As a result, today’s pasteurization standards are based on the destruction of the most heat-resistant milk-borne zoonotic agent, Coxiella burnetii [2]. C. burnetii, an obligate intracellular rickettsial bacteria, is the causative agent of Q Fever. First identified in 1935, its main reservoirs are dairy cattle, sheep, and goats. Most infected animals are asymptomatic, but chronic infections can lead to abortions and reproductive disorders in ruminants. Human infections are also often asymptomatic, but manifestations include febrile illness and malaise progressing to meningoencephalitis [4]. Shedding of C. burnetii in infected cattle occurs largely through birthing fluids, which is the main source of infection in humans [5]. However, C. burnetii can also be shed into dairy milk, nearly an exclusive route in asymptomatic herds [6,7]; shedding can be continuous or intermittent over several months and may be associated with chronic subclinical mastitis [8]. Therefore, asymptomatic cattle herds can be considered potential bacterial reservoirs, capable of transmitting Q fever through raw milk [9].
Assessing prevalence for C. burnetii in dairy cattle has been difficult and has historically documented varied results. Reported claims of national seroprevalence range from 1% to 73%, but data fluctuates annually because serology results do not correlate with shedding of C. burnetii in dairy cattle [7]. Today, bulk tank milk (BTM) samples are commonly used to check for the presence of C. burnetii using Real-time PCR assays [7,10]. This technique is considered more accurate, as a recent nationwide study estimated the dairy cattle herd-level prevalence to be around 94% over a three-year period [11]. A follow-up study in central United States estimated the prevalence to be around 77%, with a higher number of positives in larger herds [12]. Finally, a study analyzing C. burnetii prevalence in bovine raw milk available for human consumption demonstrated a 43% positivity rate with viable pathogen detected by isolation in tissue cultures; the isolation of viable C. burnetii in raw milk combined with its endemic nature and zoonotic potential suggests that raw milk consumption may pose a significant public health risk [7].
Although the pathogenicity of C. burnetii from ingestion has yet to be investigated, there are documented cases of individuals acquiring Q fever through raw milk consumption, as seen in a Michigan cluster in 2011. In this case, a few customers acquired raw milk through family members or cow-share agreements, making raw milk consumption the only plausible route of transmission. This incident highlights the risk of Q fever infection through indirect access to raw milk [4].
Despite its highly infectious nature, C. burnetii is often underassessed in dairy operations and no recent prevalence studies have been conducted in New England. Furthermore, there is little epidemiological data on the association with raw milk, or how management practices may increase or decrease the transmission. The primary aim of this study was to determine the herd-level prevalence of C. burnetii in bovine dairy operations selling raw milk and milk intended for pasteurization, also called commercial milk, in Connecticut, Rhode Island, and Massachusetts. Additionally, we aimed to assess dairy management practices and farm characteristics as factors associated with C. burnetii prevalence. Lastly, we intended to question farmers on raw milk consumption habits.
Materials and Methods
Questionnaire Survey
A total of 78 dairy producers in Connecticut (n=36), Massachusetts (n=33), and Rhode Island (n=9) consented to participate in the study between June and August 2014. A list of all registered farms, including legal status to sell raw milk, was obtained from participating state departments of agriculture and operations were selected using purposive sampling techniques. No identifying information was documented, and farms were coded for analysis. All visits were conducted on site with a veterinarian or state dairy inspector present.
A self-administered paper questionnaire survey was used to collect information on milk sale, herd size, facility age, housing, bedding, use of cooling fans, somatic cell count, milking protocols, and raw milk consumption practices. Question responses were tabulated in Microsoft Excel. For each farm characteristic, a test of significance (X2 log likelihood ratio test) was applied to determine if there was association with a positive or negative PCR test outcome. Questions regarding housing and bedding had multiple answer options, so only the frequency of positive C. burnetii tests were calculated. R (R-CRAN) was used to perform the likelihood ratios, odds ratios, and frequency calculations. Statistical significance was accepted at p < 0.05. The study protocol (#11268) was approved by Tufts Health Sciences Campus Institutional Review Board (IRB).
Sample Collection of BTM
A single bulk tank milk (BTM) sample of approximately 30 mL was collected in a sterile snap cap milk collection vial. Milk samples were collected and handled according to National Mastitis Council standards (1999). Bulk tanks were agitated based on standard manufacturer recommendations and samples were collected from the top port using a sanitized dipper. Samples were stored on ice, transported to the laboratory at Cummings School of Veterinary Medicine at Tufts University, and processed into milk pellets. Samples were centrifuged for 15 minutes at 1700g, the pellets were dried and resuspended in phosphate-buffered saline, and then centrifuged a second time. The pellets were then transferred to a 1mL polypropylene snap tube and stored at -80C. Samples were shipped on dry ice to the Cornell University Animal Health Diagnostic Center. Pellets were screened using a real-time polymerase chain reaction (Rt-PCR) assay targeting a specific transposon sequence IS1111, unique to C. burnetii (11).
Results
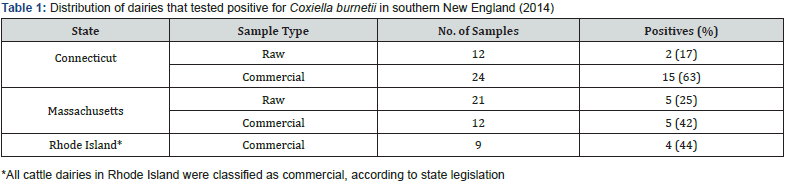
Of the 78 dairy operations in Connecticut, Rhode Island, and Massachusetts, 31 (40%) tested positive for C. burnetii, with 7 out of 33 (21%) raw dairies testing positive and 24 out of 45 (53%) commercial dairies testing positive. All suspect positives were considered true positives, acknowledging that suspect results reflect the positive PCR detection of the target sequence with low copy numbers. Results were tabulated by state and tallied in Table 1.
In this study, commercial dairies were 4.12 times more likely to test positive than raw milk dairies (p < 0.01). Herd size was a significant variable, where large dairies with greater than 100 cows were 24.3 times more likely to test positive than small dairies with less than 100 cows (p < 0.001). Analysis of these two main effects shows that there is some interaction between dairy type and herd size. The data was stratified by herd size to reassess the odds; among small herd sizes, raw and commercial dairies were equally likely to test positive for C. burnetii, while among large dairies, commercial dairies were nearly 25 times more likely to test positive than raw dairies (p < 0.05). (Figure 1).
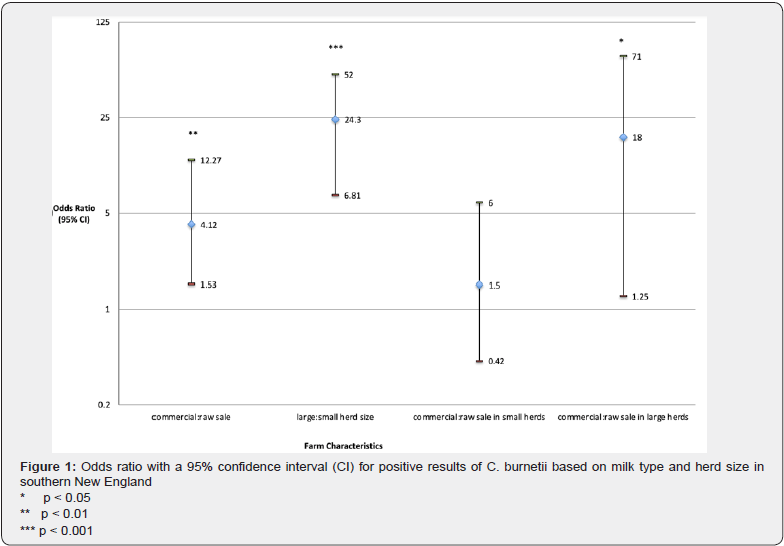
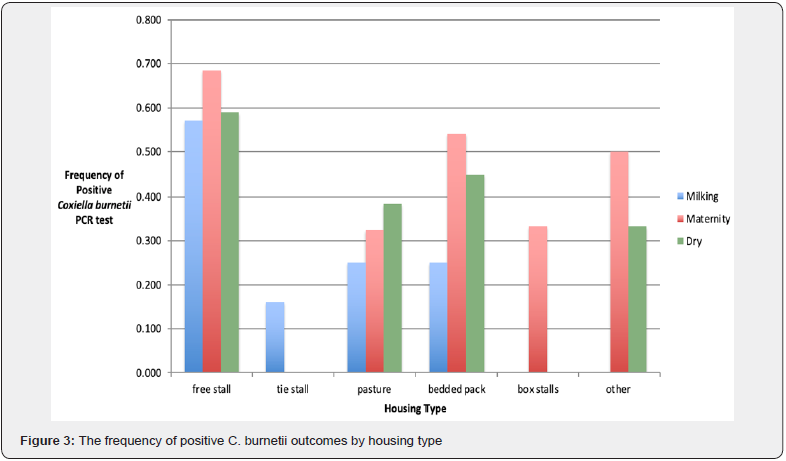
Farm management practices and characteristics were evaluated to determine the effects on prevalence (Figure 2). Farms using cooling fans were 6.7 times more likely to test positive (p < 0.001), farms where milking personnel wore gloves were 3.8 times more likely to test positive (p < 0.01), and farms with somatic cell counts greater than 200,000 cells/mL were 2.5 times more likely to test positive (p < 0.05). The use of forest ripping technique was significantly protective with lower odds of testing positive (p < 0.05). There were no significant findings for facility age or the use of pre-dip technique. The frequency of positive C. burnetii PCR tests were evaluated in the context of housing type (Figure 3) and bedding type (Figure 4) in milking cow, maternity, and dry cow barns. For housing type, free stalls had the high frequency of positive results in all three barns (p < 0.01) and for bedding type, sand bedding had the highest frequency of positive results in all three barns (p < 0.05).
Lastly, when asked about raw milk consumption habits, all 33 raw milk dairy producers (100%) and 64% of commercial dairy producers said they and/or their family members consumed raw milk from their own farm. When stratified by state, 81% of Connecticut dairy producers, 85% of Massachusetts dairy producers, and 56% of Rhode Island dairy producers said they and/or their family consumed raw milk from their own farm. Results were tabulated by producer type and state and tallied in Table 2. The most cited reasons for consuming raw milk were taste (29%) and convenience (27%). Other reasons include health benefits (23%) and cost (21%). When asked about duration of raw milk consumption, most producers reported consuming raw milk for 40 to 60 years (24%).



Discussion
Given the high prevalence rates of Coxiella burnetii found in BTM samples [12,13], the pathogen is arguably endemic to the United States. This study found a prevalence of 40% in southern New England, with 21% of raw dairy farms testing positive and 53% of commercial dairy farms testing positive. These findings are like previous estimates of C. burnetii in raw milk samples in the United States by Loftis et al. [7]. To our knowledge, this is the first study to analyze the prevalence of C. burnetii in southern New England and compare prevalence rates between raw and commercial dairies. Variations in prevalence could be due to sampling or PCR technique, seasonality, geographic area, pathogen viability, and hygiene practices not evaluated in this study. Additionally, this study only analyzed a single BTM sample for each farm; it is possible that repeated sampling over a longer period would result in a higher incidence of pathogen detection [14].
Commercial dairies were about 4 times more likely to test positive than raw milk dairies in Southern New England. Several commercial dairies had suspect positives, so further analysis of copy numbers and bacterial counts is needed in future studies. Other factors may be of influence too, such as the number of dairy farms by type and herd size. In southern New England, there are approximately 500 commercial milk dairies compared to only about 45 raw milk dairies.
Herd size was the most significant dairy farm factor evaluated. Large herd sizes were classified as exceeding 100 milking and dry cows while small herd sizes were less than 100 milking and dry cows. In this study, 91% of raw milk dairies surveyed classified themselves as small, while only 50% of commercial milk dairies classified themselves as small. Large herd sizes were 24 times more likely to test positive for C. burnetii than small herd sizes. This finding confirms previous results in both the United States and Europe, where the percentage of operations testing positive for C. burnetii increased as herd size increased (United States Department of Agriculture, 2011). Research in Scandinavia demonstrated that herds greater than 150 cows were nearly 18 times more likely to test positive than herds less than 80 cows [15]. Other cattle diseases such as Bovine Leukosis Virus (BLV), Johne’s disease, and E. coli all have higher prevalence rates as herd size increases [16].
The findings when the data stratified by herd size showed that among large herd sizes, commercial dairies had significantly higher odds of testing positive than raw milk dairies, but among small herd sizes, the two types were equally like to test positive for C. burnetii. This reaffirms that C. burnetii is prevalent in raw milk and may serve as potential health hazard for those consuming raw milk regularly from southern New England dairies. Furthermore, there is potential adverse impact in increasing herd size and seeking raw milk sale permits in southern New England states. Herd size is a crucial management practice to investigate in southern New England, considering that nearly 75% of all New England dairy farmers would be classified as small, or having less than 100 cows [17]. Although shedding in milk has not been shown to correlate with serology, further studies should evaluate any serology correlation to herd size, and the implications for cow, farmer, and veterinarian health.
Farm management practices, including the use of cooling fans and wearing gloves when milking, were found to significantly increase the odds of testing positive for C. burnetii. Use of cooling fans may aid in the spread and transmission of C. burnetii, given that it is an airborne pathogen and highly resistant to environmental stressors [5]. Farms that used cooling fans were most likely to use them in the milking barn facilities and dairies with larger herd sizes were more inclined to use cooling fans.
Farmers are highly encouraged to wear gloves to reduce rates of mastitis [8] and herds where milking personnel wear gloves are often better managed [16] Although this study found that farms that encouraged glove use had higher odds of testing positive, there may be possible unknown factors related to glove use that might influence the results, given that little is known about Coxiella burnetii transmission. These results do contradict other study findings regarding forest ripping and somatic cell count. However, there may also be a response bias for farmers to indicate that they do wear gloves, given that farmers are acutely aware of the industry recommendations for glove use.
Many farmers practice forest ripping, or initial milk stream evaluation, as part of a pre-milking udder preparation routine. Research on the value of this technique has been inconclusive, but some studies have shown that proper udder preparation including forest ripping results in greater milk yield and shorter milking times [18]. Our study found forest ripping to be protective. C. burnetii is an obligate intracellular pathogen and has been detected in neutrophils in mammary tissue. Neutrophils are likely to be found near the teat ends, so forest ripping may remove large numbers of cells that would have otherwise ended up in the bulk tanks [16].
Somatic cell count was evaluated in the context of assessing subclinical mastitis, which is classified as greater than 200,000 cells/mL [8]. In our study, farms with reported counts greater than 200,000 cells/mL were 2.5 times more likely to test positive than farms with reported counts lower than 200,000 cells/mL. This finding is significant and does support previous research that suggests an association between positive C. burnetii PCR status and chronic subclinical mastitis in lactating dairy cows. Furthermore, there may be mammary specific manifestations of C. burnetii in dairy cattle [8]. Other management practices including the age of the facility where the milking cows were housed, the use of pre-dip, and the use of post-dip technique were not found to be statistically significant. All 78 farmers acknowledged post dipping as part of their milking protocol, so this variable was not analyzed.
For housing, free stall housing had the highest frequency of positives for C. burnetii in all three locations, milking cow barn, maternity pen, and dry cow barn. Housing type has consistently been evaluated in dairy cattle lameness and claw disorder studies [19], but not in infectious disease studies. However, when looking at the setup of free stall housing, there is more movement among dairy cattle and more exposure to manure, which may allow for increased pathogen exposure. Although little is known about routes of transmission in cattle, there is some evidence that seropositive ruminants can shed C. burnetii through fecal matter [10]. Increased movement and exposure to manure is also seen in bedded pack setups, which had the second highest frequency of positives. Note that herd size may be considered a confounder or effect modifier for housing prevalence, since 85% of large diaries surveyed used free stall housing in some combination.
For bedding substrate, sand had the highest frequency of positives for C. burnetii, while hay and straw had the lowest frequency of positives. Past studies have shown that different pathogens have different prevalence rates in bedding. While E. coli and Klebsiella species were much more likely present in sawdust bedding, Streptococcal species had higher rates in sand bedding; the investigators hypothesized that the physical properties of sand, such as adhesiveness or abrasiveness, affect bacterial exposure more sporadically than sawdust [20]. Studies have also shown that cows have greater lying down times, as much as 2.3 hours per day, in sand bedding compared to sawdust bedding [21]. The choice of bedding and substrate subtypes may have implications for pathogen prevalence and transmission, warranting further investigation.
The final part of the survey inquired about farmer raw milk consumption habits. While state legislation and licensing dictate the sale of raw milk, dairy farmers are legally permitted to consume raw milk from their own farm property. All 33 raw milk dairy farmers said they and/or their family consumed raw milk while only 64% of commercial dairy farmers said they and/ or their family consumed it. The most cited reasons for raw milk consumption on the farm were taste and convenience, which supports other study findings [14]. While some surveys report onfarm raw milk consumption is highest among young men, ages 18 to 29 [14], our study found the most common age to be between 40 and 60 years, which may be simply due to regional demographics.
When analyzed by the state, 81% of Connecticut dairy farmers and 85% of Massachusetts dairy farmers said they consumed raw milk from their dairy farm. This contrasts with Rhode Island, a state that does not permit the sale of raw milk, where only 56% of dairy farmers acknowledge consuming raw milk from their dairy farm. The declining industry in New England and changing landscape in states like Rhode Island may contribute to wavering perspectives regarding raw milk consumption [17]. State and local legislation dictate the sale of raw milk and have the responsibility for milk safety, so the implications of law may also influence personal consumption habits [22]. It has been demonstrated that states that restrict raw milk sales have fewer dairy-associated outbreaks of foodborne illness [1]. Nonetheless, these findings indicate that a high number of Southern New England dairy producers and their families are at risk for exposure to C. burnetii through the consumption of raw milk.
Conclusion
Coxiella burnetii is present in dairy herds in Southern New England and regular consumption of raw milk may pose a potential health risk for Q fever infection. Certain management practices and farm characteristics were shown to increase or decrease the odds of testing positive for C. burnetii in raw milk. These findings support public health efforts to discourage the consumption of raw milk and to evaluate new methods of transmission not previously studied. Little is known about the transmission of Coxiella burnetii, and there are many interactions that are yet to be assessed. This study was limited by small sample size, timing, and availability so further review of management practices as effect modifiers and potential sources of transmission is necessary. Further studies are also warranted to determine the pathogenicity of C. burnetii and the risk of seroconversion after ingestion. C. burnetii is a select agent and CDC Category B bioterrorism agent [23] and is therefore highly regulated by the federal government; however, its endemic nature and presence in commercially available raw milk raise questions regarding the feasibility and effectiveness of such control efforts, as well as the implications for outbreaks and bioterrorism events. Despite its ubiquity and high zoonotic potential, Coxiella burnetii is typically not pathogenic in cows, making diagnosis and prevention difficult. However, it has been detected in bulk tank milk of raw and commercial dairy herds, and because foodborne illness from raw milk consumption is preventable, pasteurization along with education are critical public health tools. Improving awareness among farmers and veterinarians regarding C. burnetii prevalence and routes of transmission is important for prevention, detection, and later diagnosis. This knowledge can also improve dairy management practices for cow health and biosecurity.
Acknowledgments
The author would like to acknowledge the support of staff and faculty at the Cummings School of Veterinary Medicine and School of Medicine at Tufts University for their assistance in study design and review. The members of Connecticut Department of Agriculture, Massachusetts Department of Agriculture, Rhode Island Department of Environmental Management, and field staff at Agri-Mark are also recognized for their assistance in data collection. Veterinarians at the Cornell University Animal Health Diagnostic Center are recognized for their involvement with materials and methods. Finally, the dairy producers who participated in this study are recognized with gratitude. This study was funded in part by the NIH Short-Term Training Grant and the Morton A. Madoff Public Health Fellowship from Tufts University School of Medicine.
References
- Langer AJ, Ayers T, Grass J, Lynch M, Angulo FJ, et al. (2012) Non-Pasteurized Dairy Products, Disease Outbreaks, and State Laws, 1993–2006. Emerg Infect Dis 18: 385-391.
- Claeys WL, Cardoen S, Daube G, Block JD, Dewettinck K, et al. (2013) Raw or Heated Milk Consumption: Review of Risk and Benefits. J Food Control 31: 251-262.
- Food and Drug Administration (USA) (2012) The Dangers of Raw Milk: Unpasteurized Milk Can Pose a Serious Health Risk. Food Facts P: 1-2.
- Signs KA, Stobierski MG, Gandhi TN. (2012) Q Fever Cluster Among Raw Milk Drinkers in Michigan, 2011. Brief Report CID. 55: 1387-1389.
- Anderson A, Bijlmer H, Fournier PE, Graves S, Hartzell J, et al. (2013) Diagnosis and management of Q Fever-United States. Centers for Disease Control and Prevention MMWR 62(3): 1-32.
- Rodolakis A, Berri M, Hechard C, Caudron C, Souriau A, et al. (2007) Comparison of Coxiella burnetii Shedding in Milk of Dairy, Bovine, Caprine, and Ovine Herds. J Dairy Sci 90: 5352–5360.
- Loftis AD, Priestley RA, Massung RF (2010) Detection of Coxiella burnetii in Commercially Available Raw Milk from the United States. Foodborne Patho and Dis 7: 1453-1456.
- Barlow, J, Rauch B, Welcome F, Kim SG, Dubovi E, et al. (2008) Association between Coxiella burnetii shedding in milk and subclinical mastitis in dairy cattle. Vet Res 39: 23-31.
- Rodolakis A (2009) Q Fever in Dairy Animals. Ricketts ology And Rickettsial Diseases - Fifth International Conference. New York Academy of Sciences 1166: 90-93.
- Berri, M, Laroucau K, Rodolakis A (2000) The detection of Coxiella burnetii from ovine genital swabs, milk and Fecal Samples Using a Single Touchdown Polymerase Chain Reaction. Vet Microbiol 72: 285-293.
- Kim SG (2013) Bovine infection of Coxiella burnetii (Q fever) in the U.S. dairy herds: use of conventional and real-time PCR for the detection of Coxiella burnetii (Q fever) in milk. Molecular Methods for Milk Quality – Cornell University Animal Health Diagnostic Center pp: 86-87.
- Kim SG, Kim EH, Lafferty CJ, Dubovi E (2005) Coxiella burnetii in bulk tank milk samples, United States. Emerg Infect Dis 11: 619-621.
- USDA APHIS Veterinary Services (2011) Prevalence of Coxiella burnetii in Bulk-tank Milk on U.S. Dairy Operations, 2007. APHIS Technical Brief p: 1-2.
- Jayarao BM, Donaldson SC, Straley BA, Sawant AA, Hegde NV, et al. (2006) A Survey of Foodborne Pathogens in Bulk Tank Milk and Raw Milk Consumption Among Farm Families in Pennsylvania. J Dairy Sci 89: 2451-2458.
- Agger, JF, Suman P (2014) Increasing prevalence of Coxiella burnetii seropositive Danish dairy cattle herds. Acta Veterinaria Scandinavica 56: 46-49.
- Divers TJ, Peek SF (2008) Rebhun's Diseases of Dairy Cattle. Saunders Elsevier 2nd Pp: 621-623.
- Horwitz, RP (2011) Foot-and-Mouth (FMD) as a Hazard for New England Dairies. U.S. Department of Agriculture, New England States Animal Agricultural Security Alliance with APHIS Cooperative Agreement # 10-9623-1062 (BCOP, FFY 2010). Pp: 35-44 & Pp:75-83.
- Sandrucci A, Tamburini A, Bava L, Zucali M (2007) Factors Affecting Milk Flow Traits in Dairy Cows: Results of a Field Study. J Dairy Sci 90(3): 1159-1167.
- Cook NB (2013) Prevalence of lameness among dairy cattle in Wisconsin as a function of housing type and stall surface. J. Am. Vet Med Assoc 223(9): 1324-1328.
- Zdanowicz M, Shelford JA, Tucker CB, Weary DM, von Keyserlingk MAG (2004) Bacterial populations on teat ends of dairy cows housed in free stalls and bedded with either sand or sawdust. J Dairy Sci 87(6): 1694-1701.
- Drissler M, Gaworski MA, Tucker CB, Weary DM (2005) Freestall maintenance: effects on lying behavior of dairy cattle. J Dairy Sci 88(7):2381-2387.
- Hendrick S, Farquhar D (2013) Summary of Raw Milk Statutes and Administrative Codes. National Conference of State Legislatures, United States Pp: 1-88.
- Centers for Disease Control and Prevention (USA) (2018) Bioterrorism Agents/Diseases.






























