Histoenzymic Localization of Phosphatases and Oxidoreductases in the Tonsils of Oro-pharyngeal Region of Buffaloes (Bubalus bubalis)
Pawan Kumar*, Amandeep Singh, Parveen Kumar Gahlot and Tej Parkash
Department of Veterinary Anatomy, College of Veterinary Sciences, Lala Lajpat Rai University of Veterinary and Animal Sciences, India
Submission: June 08, 2023; Published: July 03, 2023
*Corresponding author: Pawan Kumar, Department of Veterinary Anatomy, College of Veterinary Sciences, Lala Lajpat Rai University of Veterinary and Animal Sciences, Hisar-125 004, India. E-mail: pawanrajoria2000@rediffmail.com,pkumar@luvas.edu.in
How to cite this article: Pawan K, Amandeep S, Parveen Kumar G, Tej Parkash. Histoenzymic Localization of Phosphatases and Oxidoreductases in the Tonsils of Oro-pharyngeal Region of Buffaloes (Bubalus bubalis). Dairy and Vet Sci J. 2023; 15(5): 555923.DOI: 10.19080/JDVS.2023.15.555923
Abstract
The present study was envisaged to explore the localization of a panel of oxidoreductases and phosphatases enzymes in the lingual tonsil, palatine tonsil, and tonsil of soft palate of six healthy adult buffaloes. The succinic dehydrogenase, lactic dehydrogenase, glutamic dehydrogenase, and glucose 6-phosphate dehydrogenase showed a strong positive activity in the stratum Basale layer of the stratified squamous epithelium of the tonsils. The diffuse lymphoid tissue and the striated muscles showed moderate to strong positive reactions. The mucous secretory acini did not show any affinity for these enzymes, however, a uniform moderate to strong positive reaction was observed towards their basement membranes. The acid phosphatase, alkaline phosphatase, and glucose-6- phosphatase showed a strong positive activity in the deepest layer of cells next to the basement membrane of the stratified squamous epithelium of all the tonsils of oro-pharyngeal region. The associated submucosal lymphoid tissue and the glandular tissue of the tonsils showed positive reactions like those of the oxidoreductases group of enzymes. A comparison of the pattern of localization of these enzymes in healthy and infected animals may play a significant role in disease diagnosis, if any.
Keywords: Buffalo; Oxidoreductases; Phosphatases; Tonsils
Keywords: SDH: Succinic dehydrogenase; LDH: Lactic dehydrogenase; GLD: Glutamic dehydrogenase; G-6-PD: Glucose-6-phosphate dehydrogenase; ACP: Acid phosphatase; AKP: Alkaline phosphatase; G-6-Pase: Glucose-6-phosphatase
Introduction
Tonsils, part of the mucosa-associated lymphoid tissue, are located at defined mucosal sites and form a first line of defense against foreign antigens. The tonsils of the oro-nasopharyngeal region constitute Waldeyer’s ring [1], playing a key role in initiating immune responses against antigens entering the body through the oral route [2]. The tonsils of the oro-pharyngeal region (lingual tonsils, palatine tonsils, and tonsils of the soft palate) are covered with stratified squamous epithelium [3-6], whereas those of the nasopharyngeal region (nasopharyngeal and tubal tonsils) are lined with pseudostratified columnar epithelium [7,8]. Histological features like active germinal centres, the presence of plasma cells, follicular dendritic cells, and acid phosphatase-positive antigen-presenting cells indicate the immune competency of the lymphoid organs [9]. The tonsils acted as replication sites for some pathogens and played a significant role in the pathogenesis of several infectious diseases of cattle, especially prions causing bovine spongiform encephalopathy [10,11]. Extensive research has been conducted on the tonsils of buffaloes, except for histoenzymology. Enzyme histochemistry serves as a link between biochemistry and morphology, and their varying levels may be used as biochemical markers indicating the health status of the animal. Oxidoreductases, a group of enzymes, are actively involved in the physiological process of respiratory chain pathway due to their capability of oxido-reduction reactions. Similarly, phosphatases enzymes play key role in cellular functions due to phosphorylation and dephosphorylation. The present study is the first of its type to demonstrate the qualitative distribution of oxidoreductases and phosphatases group of enzymes in the lingual tonsil, palatine tonsil, and the tonsil of soft palate of buffaloes. The localization of these enzymes in different tonsils of buffalo may be correlated with physiological functions and may be useful for planning research projects associated with oral vaccination programs.
Materials and Methods
The fresh tissues from the lingual, palatine, and tonsil of soft palate of six adult healthy buffaloes of either sex of 5-6 years age of local mixed breed were collected immediately after their sacrifice by captive bolt stunning gun method at the slaughterhouse, New Delhi. An approval of the Institutional Animal Ethical Committee was not required as the collection was done after the death of the buffaloes. The tissues collected in liquid nitrogen were stored at -20°C, and later blocks were made using tissue freezing medium. The frozen sections of 10-12 μ thickness cut with cryostat microtome at -20°C were mounted on clean glass slides coated with 3-aminopropyltriethoxy-silane. The sections defrosted at room temperature were circled with a PAP pen slide marker and incubated with different substrates at 37°C to study the distribution pattern of different oxidoreductases and phosphatases enzymes (Table 1).
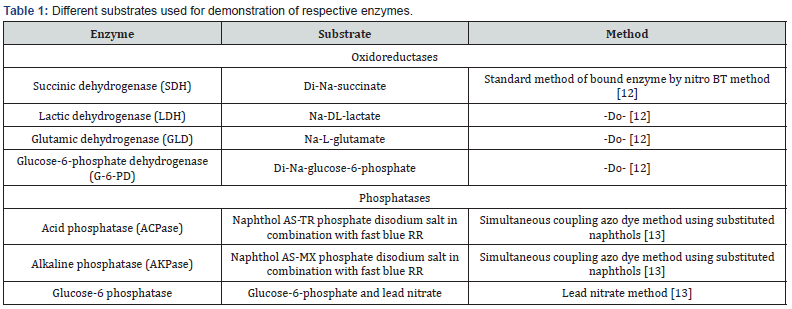
Results and Discussion
A detailed light histological, histochemical, and electronmicroscopic study has been carried out on the tonsils of buffaloes [3-8]. A lack of related literature on the histoenzymology of the tonsils of the animals was the main limitation to compare and discuss the present findings with those of previous reports; however, these specific localizations in healthy animals can be later utilized as markers in different disease conditions.
Succinic dehydrogenase (SDH)
A strong uniform positive granular reaction was observed in the stratum Basale layer of the stratified squamous epithelium of all the tonsils i.e., lingual, palatine and the tonsil of soft palate (Figure 1 A,B,C) whereas; the rest of strata were devoid of positive activity except the most superficial layers where a moderate reaction was observed. The subepithelial connective tissue had no affinity for the enzyme. The blood vessels showed moderate positive reaction. The diffuse submucosal lymphoid tissue showed uniform weak to moderate reaction in the form of granular activity for the enzyme. The secretory glandular acini and their ducts showed a negative reaction. However, a uniform moderate positive activity was seen towards their peripheral portions in the region of myoepithelial cells. A strong intense positive reaction was observed in the striated muscles present in the deeper portion of the propria submucosa. The rest of the connective tissue was nonreactive towards the enzyme. The SDH enzyme found in all the aerobic cells is an essential part of the Krebs cycle. This enzyme, a mitochondrial enzyme, related to mitotic and secretory activity is involved in oxidation [12,13].
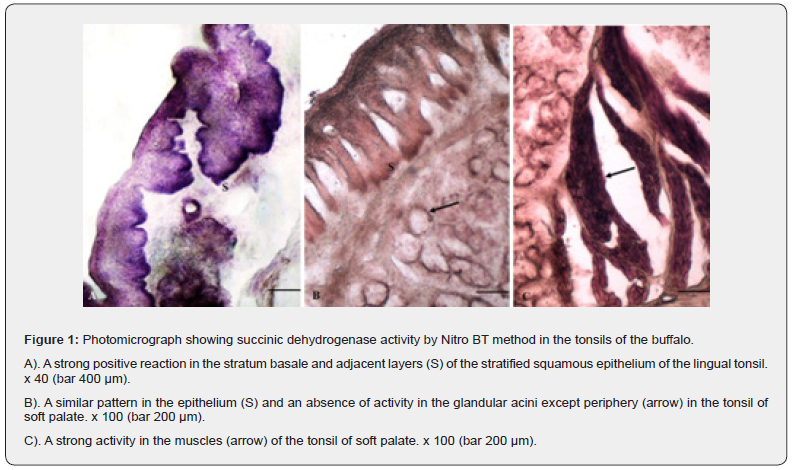
Lactic dehydrogenase (LDH)
The stratified squamous epithelium of all the oral tonsils (Figure 2A, B) showed mild to moderate reaction for the enzyme in different strata except the stratum Basale where a strong evenly distributed activity was observed. The pattern of reaction was similar in all the three types of tonsils. A negative reaction was seen in the subepithelial portion of the connective tissue. The lymphoid aggregates presented moderate to strong affinity for the enzyme. The reactivity for the enzymes was slightly stronger towards the periphery as compared to the central part of the lymphoid tissue indicating a higher concentration in T-lymphocytes than the B-cells. The glandular tissue devoid of the positive activity showed presence of the enzyme towards the basement membrane of the glands. The muscular tissue (Figure 2C) interspersed between the interlobular connective tissue and the blood vessels of varying sizes exhibited moderate positive reaction. LDH is a membrane bound enzyme present in almost all the organs released after injury in response to the inflammation and may be considered as a diagnostic reflection of infection as detected in nasopharyngeal secretions after upper respiratory affections and otitis media [14]. Higher concentration of the enzyme has been reported in cases affected with adeno and rhinoviruses and showed a positive correlation with an increased level of cytokines [15]. The extracellular LDH is not present under normal conditions so it may be used as a biomarker tool for cellular injury. The injury to tonsil may occur from a multitude of factors including direct virusinduced cytopathic injury of the infected cells leading to release of chemokines and cytokines which act on local endothelium and epithelium to enhance the migration of leukocytes towards infected epithelial cells [15]. The presence of enzyme indicated an anaerobic cellular respiration and involvement in an increased glycolytic pathway protein synthesis in active cells [16].
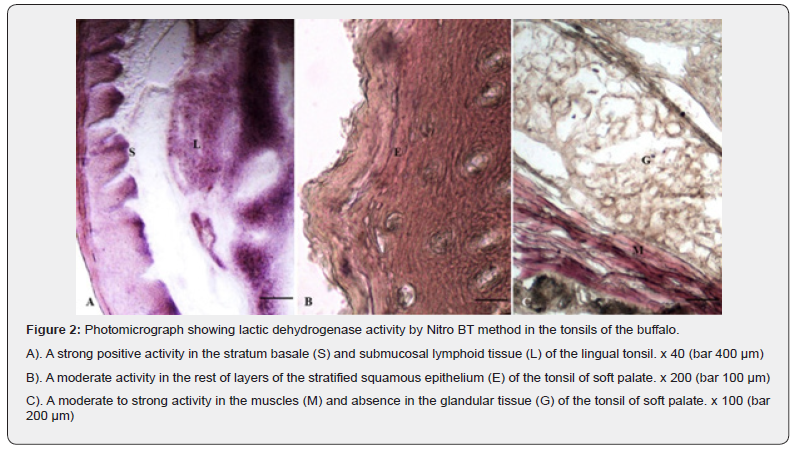
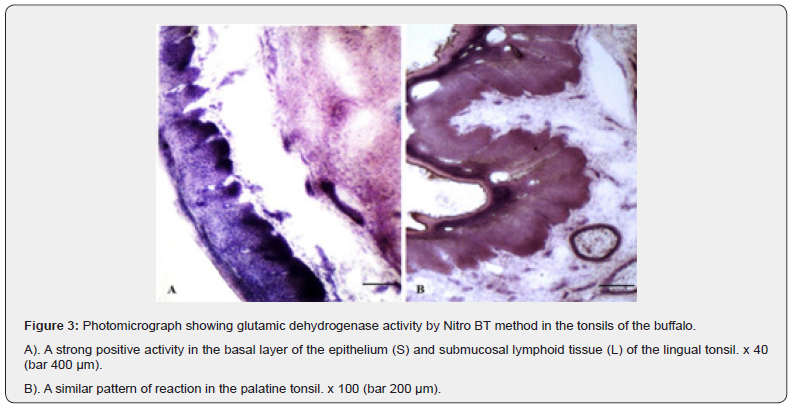
Glutamic dehydrogenase (GLD)
The stratum Basale layer of the epithelium of tonsils of Oropharyngeal region exhibited an evenly distributed strong positive activity for GLD whereas; the most superficial layers showed moderate positive granular reaction. The rest of layers were negative for the presence of the enzymatic reaction (Figure 3 A, B). The lymphoid tissue showed moderate to strong positive reactions whereas; the striated muscles distributed in between the glandular tissue and in the deeper portion exhibited strong positive activity for the enzyme. The glandular acini were free from the positive reaction except their basal portions having moderate affinity. The blood vessels and glandular ducts also showed moderate to strong positive reactions for the GLD.
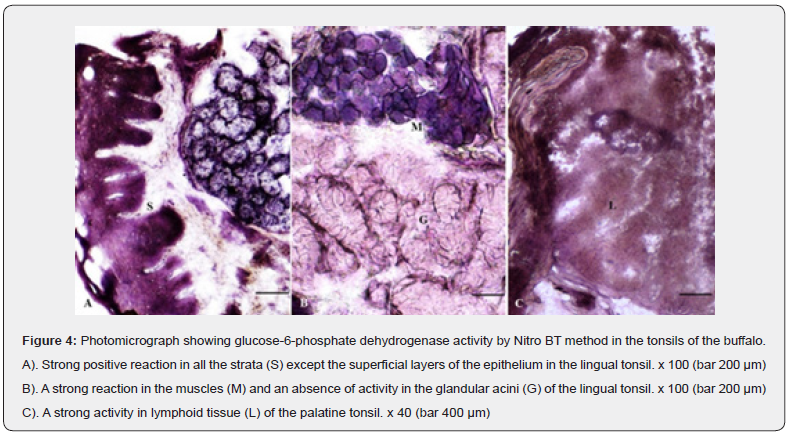
Glucose-6-phosphate dehydrogenase (G-6-PD)
A weak to moderate uniform glucose-6-phosphate dehydrogenase positive granular reaction observed in all the strata of the stratified squamous epithelium was stronger in the basal layers (Figure 4A). The activity for the enzyme was absent in the subepithelial connective tissue and the secretory acinar cells excepting their peripheral portions which presented a moderate to strong activity (Figure 4 A, B). The blood vessels and diffuse lymphoid tissue showed moderate to strong positive reaction (Figure 4C). A uniform strong positive reaction was observed in the striated muscles present in the deeper part of the propriasubmucosa (Figure 4B). A higher concentration of the enzyme was suggestive of more utilization of glucose or an increased protein synthesis [17].
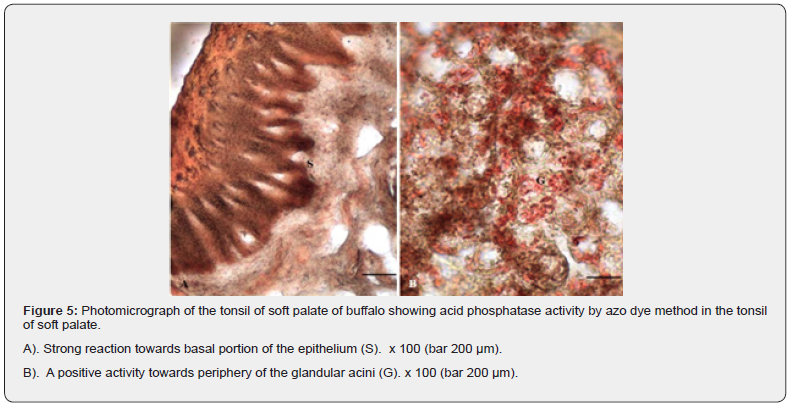
Acid phosphatase (ACP)
The enzyme was mainly localized in the basal and most superficial layers of the tonsillar epithelium (Figure 5A). The enzyme showed moderate activity in the rest of the layers of the epithelium and the lymphoid tissue. A weak reaction was also observed in the subepithelial connective tissue. The glandular acini presented a moderate to strong positive reaction (Figure 5B). The acid phosphatase enzyme, in tonsillar tissues, characteristic of the follicular dendritic cells and macrophages may be considered as a marker for the latter adjacent to the T cells [18].
Alkaline phosphatase (AKP)
Alkaline phosphatase enzyme could not be demonstrated in the tonsils during the present study. The presence of AKP in the epithelium may be correlated with the transportation of ions across the membrane with high physiological activity [19] and used as a specific marker for the B cells activation [20]. However, the expression of the enzyme has been correlated with differentiation of B cells and IgM secretions [21]. A subset of B cells in the subepithelial portion of the tonsil was also positive for the enzyme and the area may be having a specific role in activation of B cells [22]. The lymphocytes present in the germinal centers showed varying positive to negative reactions indicating some physiological differences in these cells. In addition, the conversion of lymphocytes into macrophages involves cellular energy which might be provided by alkaline phosphatase enzyme [23]. Tissue non-specific alkaline phosphatase enzyme is a cell membrane bound glycoprotein that may be involved in transmitting the information between different types of immunologic cells and mainly expressed in the B cells leading to their differentiation, activation, and proliferation [24].
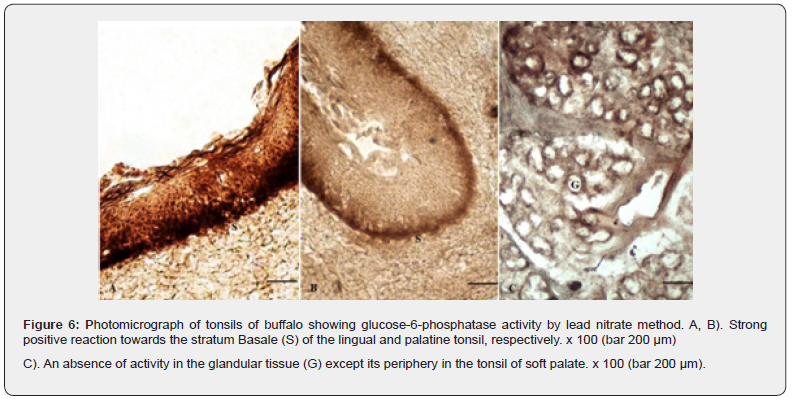
Glucose-6-phosphatase (G-6-Pase)
A strong positive G-6-Pase enzyme activity was observed in the stratum basale of the epithelium (Figure 6). The activity was weak to absent in the rest of the epithelial layers. The enzyme is a membrane-bound enzyme and has been found to be firmly associated with endoplasmic reticulum and related to carbohydrate metabolism [25]. The submucosal connective and the associated lymphoid tissue showed negative and weak positive reactions, respectively. The glandular acini exhibited localization of the enzyme only towards the basement membrane and myoepithelial cells (Plate 6 C). The striated muscle fibres were also negative for the enzyme activity. A strong positive reaction observed in the active cells has been correlated with a higher concentration of the endoplasmic reticulum [26].
Conclusion
A strong affinity of the basal layers of the epithelium for all the enzymes (oxidoreductases and phosphatases) indicated their importance in the maintenance and the growth of the epithelial cells. The present qualitative localization of these enzymes in different structural components of the tonsils may be a benchmark in health and disease conditions.
Acknowledgement
The authors gratefully acknowledge the Haryana State Council for Science and Technology, India for providing the research grant vide HSCST/R&D/2018/2101 dt. 01.08.2018.
References
- Zidan M, Pabst R (2011) The microanatomy of the palatine tonsils of the buffalo (Bos bubalus). Veterinary Immunology and Immunopathology 139: 83-89.
- Brandtzaeg P (2015) Immunobiology of the tonsils and adenoids. Mucosal Immunology 2: 1985-2016.
- Girgiri IA, Kumar Pawan (2018) Histology, histochemistry and scanning electron microscopy of tonsil of the soft palate of buffalo (Bubalus bubalis). Indian Journal of Veterinary Anatomy 30: 58-63.
- Girgiri IA, Kumar Pawan (2018) Light microscopic studies on the palatine tonsil of buffalo (Bubalus bubalis). Veterinary Research International 6: 60-66.
- Girgiri IA, Kumar Pawan (2019) Histological and histochemical studies on the lingual tonsil of the buffalo (Bubalus bubalis). Journal of Buffalo Science 9: 5-12.
- Girgiri IA, Kumar Pawan (2020) Scanning and transmission electron-microscopic studies on the lingual tonsil of the buffalo (Bubalus bubalis). Journal of Buffalo Science 8: 68-76.
- Girgiri IA, Kumar Pawan (2019) Histology, histochemistry and ultrastructure of the nasopharyngeal tonsil of the buffalo (Bubalus bubalis). Anatomia Histologia Embryologia 48: 375-383.
- Girgiri IA, Kumar Pawan (2020) Light and electron-microscopic studies on the tubal tonsil of the buffalo (Bubalus bubalis). Journal of Buffalo Science 9: 60-70.
- Ozbilgin MK, Polat SA, Mete UO, Tap O, Kaya M (2004) Antigen-presenting cells in the hypertrophic pharyngeal tonsils: a histochemical, immunohistochemical and ultrastructural study. Journal of Investigational Allergology and Clinical Immunology 14(4): 320-328.
- Liebler Tenorio EM, Pabst R (2006) MALT structure and function in farm animals. Veterinary Research 37(3): 257-280.
- Donaldson DS, Kobayashi A, Ohno H, Yagita H, Williams IR, et al. (2012) M-cell depletion blocks oral prion disease pathogenesis. Mucosal Immunology 5(2): 216-225.
- Pearse AGE (1972) Histochemistry: Theoretical and Applied. 3rd ed. Churchill Livingstone, London.
- Barka T, Anderson PJ (1963) Histochemistry: Theory, Practice and Bibliography. 1st ed. Harper and Row Publishers Inc, New York.
- Mansbach JM, Piedra PA, Laham FR, McAdam AJ, Clark S, et al. (2012) Nasopharyngeal lactate dehydrogenase concentrations predict bronchiolitis severity in a prospective multicenter emergency department study. Pediatric Infectious Disease Journal 31(7): 767-769.
- Ede LC, O’Brien J, Chonmaitree T, Han Y, Patel JA (2013) Lactate dehydrogenase as a marker of nasopharyngeal inflammatory injury during viral upper respiratory infection: implications for acute otitis media. Pediatric Research 73(3): 349-354.
- Raekallio J (1970) Enzyme histochemistry of wound healing. Progress in Histochemistry and Cytochemistry 1(2): 1-92.
- Darzi MM, Roy KS, Sood N, Gupta PP (1998) Histochemical and histoenzymic studies on caprine mammary gland following induced Mycoplasma F38 mastitis. Indian Journal of Animal Sciences 68(4): 313-316.
- Jesic S, Stojiljkovic L, Petrovic Z, Djordjevic V, Nesic V, et al. (2006) Alteration of adenoid tissue alkaline and acid phosphatase in children with secretory otitis media. International Journal of Pediatric Otorhinolaryngology 70(6): 1069-1076.
- Roy KS, Sood N, Gupta PP (1997) Enzyme histochemistry of mammary gland of goat (Capra hircus). Indian Journal of Animal Sciences 67: 772-773.
- Feldbush TL, Lafrenz D (1991) Alkaline phosphatase on activated B cells. Characterization of the expression of alkaline phosphatase on activated B cells. Kinetics and membrane anchor. Journal of Immunology 147(11): 3690-3695.
- Burg DL, Feldbush TL (1989) Late events in B cell activation. Expression of membrane alkaline phosphatase activity. Journal of Immunology 142(2): 381-387.
- Dono M, Burgio VL, Tacchetti C, Favre A, Augliera A, et al. (1996) Subepithelial B cells in the human palatine tonsil. I. Morphologic, cytochemical and phenotypic characterization. European Journal of Immunology 26(9): 2035-2042.
- Rakhawy MT, Tarkhan AA, Zakaria AM (1976) Alkaline phosphatase activity in the human tonsils and its relation to tonsillar diseases. Cells Tissues Organs 95(3): 434-443.
- Jesic S, Stojiljkovic L, Stosic S, Nesic V, Milovanovic J, Jotic A (2010) Enzymatic study of tonsil tissue alkaline and acid phosphatase in children with recurrent tonsillitis and tonsil hypertrophy. Int J Pediatr Otorhinolaryngol 74(1): 82-86.
- Chayen J, Butcher RG, Bitensky L, Poulter LW (1969) A Guide to Practical Histochemistry. Oliver and Boyl, Edinburg England.
- Shugyo Y, Watanabe J, Kanamura S, Kanai K (1986) Glucose-6-phosphatase activity in pregnant and lactating mammary glands of the mouse. Anatomical Record 214(4): 383-388.






























