The Heightened Risk of Antibiotic Resistance Prompted the Development of an Alternative Drug Target on DNA Replication
Temesgen Kassa Getahun*
Ethiopian Institute of Agricultural Research Holeta, Animal Health Research National program, Ethiopia
Submission: March 01, 2023; Published: April 04, 2023
*Corresponding author: Temesgen Kassa Getahun, Ethiopian Institute of Agricultural Research Holeta, Animal Health Research National program, Ethiopia
How to cite this article: Temesgen K G. The Heightened Risk of Antibiotic Resistance Prompted the Development of an Alternative Drug Target on DNA Replication. Dairy and Vet Sci J. 2023; 15(4): 555916.DOI: 10.19080/JDVS.2023.15.555916
Abstract
Chemical substances known as antibiotics act to inhibit the division of bacteria (a process known as bacteriostasis) or to kill the bacteria (bactericidal). Antibiotics are therefore a crucial part of medicine that is utilized to protect both human and animal health. However, the widespread use, misuse, and overuse of antibiotics in humans and animals has raised concerns about the development of antibiotic-resistant bacteria, which could endanger both animals and humans. Antibiotic resistance is brought on by several causes, including antibiotic residue in animal products, contaminated environments and water sources, the use of antibiotics in subtherapeutic doses, non-laboratory-oriented therapy, and inadequate storage. Bacteria that are resistant to antibiotics do so through a variety of processes. It is possible for zoonotic infections caused by resistant strains to happen and endanger human health. One of the most alarming consequences of this flawed system is the failure to develop new antibiotics to combat drug resistant bacteria. One of the current drug targets to combat antibiotic resistance is DNA replication. The replisome is very much an under-explored target for drug development, however, and few attempts to discover specific inhibitors have been described. There are guidelines in both human and Veterinary Medicine for rational use of antibiotics. But there are still some indications of the misuse of antibiotics by health care providers, unskilled practitioners, and drug consumers. Thus, awareness creation should be conducted on rational use of antibiotics in veterinary and medical practice to mitigate the occurrence of antibiotic resistance and for the more novel antibiotic development must be given attention. Therefore, the objective of this seminar paper is to review and summarize available information on antibiotic resistance and recent drug developments on DNA replication.
Keywords: Antibiotics; Antibiotics Resistance; Public Health Significance; Mechanism of Resistance; Recent Drug Target
Abbreviations: WHO: world health organization; PBA: Penicillin Binding Proteins; PABA: Para-Amino-Benzoic Acid; AMR: Antimicrobial resistance; ABR: Antibiotic Resistance; FDA: Food-Producing Animals; GIT: gastrointestinal tract; API: Active Pharmaceutical Ingredient; MGEs: Mobile Gene Element; MRSA: methicillin resistant Staphylococcus aureus; CATs: Chloramphenicol Acetyltransferases; MFS: major facilitator superfamily; SMR: Small Multidrug Resistance Family; RND: Resistance-Nodulation-Cell-Division Family; ABC: ATP-Binding Cassette Family; MATE: Multidrug And Toxic Compound Extrusion Family;
Introduction
An antibiotic is a type of antimicrobial substance active against bacteria and is the most important type of antimicrobial agent for fighting bacterial infections. Antibiotic medications are widely used in the treatment and prevention of such infections. They may either kill or inhibit the growth of bacteria. A limited number of antibiotics also possess antiprotozoal activity. Antibiotics are mainly used both in human and veterinary medicine to insure human and animal health worldwide. Antibiotics in humans are used to treat or prevent bacterial infections, and sometimes protozoan infections. When an infection is suspected of being responsible for an illness, but the responsible pathogen has not been identified, an empiric therapy is adopted. This involves the administration of a broad-spectrum antibiotic based on the signs and symptoms presented and are initiated pending laboratory results that can take several days. When the responsible pathogenic bacterium is already known or has been identified, definitive therapy can be started. This will usually involve the use of a narrow-spectrum antibiotic Flowers & Rollins [1,2].
Antibiotic in livestock is used for any purpose in the husbandry of livestock, which includes treatment when ill (therapeutic), treatment of a herd of animals when at least one is diagnosed as ill (metaphylaxis), preventative treatment (prophylaxis), and sub- therapeutic doses in animal feed and water to promote growth and improve feed efficiency. Besides medical therapy, antibiotics have also been used to improve aquaculture production Joshi [3]. Antibiotics given as a preventive are usually limited to at-risk populations such as those with a weakened immune system, those taking immunosuppressive drugs, cancer patients, and those having surgery. Their use in surgical procedures is to help prevent infection of incisions Bow [4].
Antibiotics that are used in humans to treat or prevent certain diseases either belong to the same general classes or have the same mode of action as those used for animals Joshi [3]. These antibiotics, despite their effectiveness, can leave residues in the treated animal and contaminate its edible parts; the muscle meat and milk Zeina [5]. The residues occur mostly when animals are slaughtered and milked, and egg lay within the withdrawal period of the drug. The presence of residues of antibiotics in meat, milk, egg, and fish has a great public health concern. Human consumption of these may be associated with the development of antibiotic resistance in human pathogens that are difficult to treat with the commonly available antibiotics Alla [6]. They range from direct toxicity on consumers exhibiting allergic reactions to indirect problems through the generation of resistant strains of pathogenic bacteria and the residual contamination of manures used in crop productions. They result in adverse public health effects including hypersensitivity, tissue damage, gastrointestinal disturbance, and bacterial resistant strain Dubois [7].
Some studies on bacterial resistance have shown that there is a huge diversity of resistance mechanisms, in which the distribution and interaction is mostly complex and unknown. However, there are varieties of biochemical and physiological mechanisms that are responsible for the development of antibiotic resistance. The mechanism of resistance may be evolution of either genetically inherent or the result of the microorganism being exposed to antibiotics. Most of the antibiotic resistance has emerged because of mutation or through transfer of genetic material between microorganisms. Several of various recent studies revealed that almost 400 different bacteria have demonstrated about 20,000 possible resistant genes Davies & Davies [8].
The resistances that evolve within bacteria that affect animals have the potential to affect humans. Zoonosis of resistant strains can occur, posing a risk to human health. People who are employed at farms or food animal production facilities are at a higher risk of infection with a resistant strain of bacteria Butler [9]. Antibiotic resistant infections occur too often and with increasing frequency, interfering with the effective treatment of people and animals. Antibiotic resistance has increased due to the introduction of antibiotics into the environment. In general practice, there are concerns about some common infections which are becoming difficult to treat an illness with antibiotic resistant bacteria which may take longer to resolve Sarmah [10]. To preserve the effectiveness of antibiotics, it is critical to examine the uses of these drugs, in both humans and animals. Several new initiatives are being put in place to halt the alarming trend of resistance to antibiotics and to deal with the ever-increasing number of infections caused by resistant bacteria CDC [11].
Report of world health organization WHO [12], on global surveillance of antibiotic resistance reveals that antibiotic resistance is no longer a prediction for the future; it is happening right now, across the world, and is putting at risk the ability to treat common infections in the community, hospitals, animals, and wildlife. The emergence of antibiotics resistance could result in the misuse of antibiotics both in humans and animals. As a result, it may lead to increased mortality, morbidity, and costs of treatment in humans and animals. There were various indications on the misuse of antibiotics by health care providers, unskilled practitioners and drug consumers that results in drug residue and there is still inadequate awareness as well as surveillance on the control and prevention of antibiotic resistance. Concomitantly some bacteria also develop resistance to all antibiotics. One of the most alarming consequences of this flawed system is the failure to develop new antibiotics to combat antibiotic resistant bacteria and the problem of antimicrobial resistance calls attention to the pressing need to reform our outdated model of pharmaceutical innovation.
Therefore, the objectives of this seminar paper are:
a) To review antibiotic resistance and its public health concern.
b) To review recent drug development against resistance to DNA replication.
Antibiotic Resistance and its Mechanism of Development
Antibiotics
Definition: An antibiotic is a type of antimicrobial drug used to treat bacterial infections (Figure1). An antimicrobial is an agent that kills microorganisms or stops their growth. Antimicrobial medicines can be grouped according to the microorganisms they act primarily against. For example, antibiotics are used against bacteria and antifungals are used against fungi. They can also be classified according to their function. Agents that kill microbes are called microbicidal, while those that merely inhibit their growth are called biostatic. The use of antimicrobial medicines to treat infection is known as antimicrobial chemotherapy, while the use of antimicrobial medicines to prevent infection is known as antimicrobial prophylaxis McDermott [13].
The main classes of antimicrobial agents are disinfectants (nonselective antimicrobials such as bleach), which kill a wide range of microbes on non-living surfaces to prevent the spread of illness, antiseptics (which are applied to living tissue and help reduce infection during surgery), and antibiotics (which destroy microorganisms within the body). The term antibiotic originally described only those formulations derived from living microorganisms but is now also applied to synthetic antimicrobials, such as the sulphonamides, or fluoroquinolones Xu [14]. The term also used to be restricted to anti bacteria’s (and is often used as a synonym for them by medical professionals and in medical literature), but its context has broadened to include all antimicrobials. Antibacterial agents can be further subdivided into bactericidal agents, which kill bacteria, and bacteriostatic agents, which slow down or stall bacterial growth. In response, further advancements in antimicrobial technologies have resulted in solutions that can go beyond simply inhibiting microbial growth. Instead, certain types of porous media have been developed to kill microbes on contact Xu [14].
History: The first commercially available antibacterial was Prontosil, a sulfonamide developed by the German biochemist Gerhard Domagk in the 1930s. Before this, in 1928, Alexander Fleming had discovered the first antibiotic, penicillin, but it took over a decade before penicillin was introduced as a treatment for bacterial infections. This was possible through the work of Florey and Chain who managed to efficiently purify the antibiotic and scale-up production. The introduction of penicillin marked the beginning of the so-called “golden era” of antibiotics. Between 1940 and 1962, most of the antibiotic classes we use as medicines today were discovered and introduced to the market. Each class typically contains several antibiotics that have been discovered over time or are modified versions of previous types of Yousef [15].
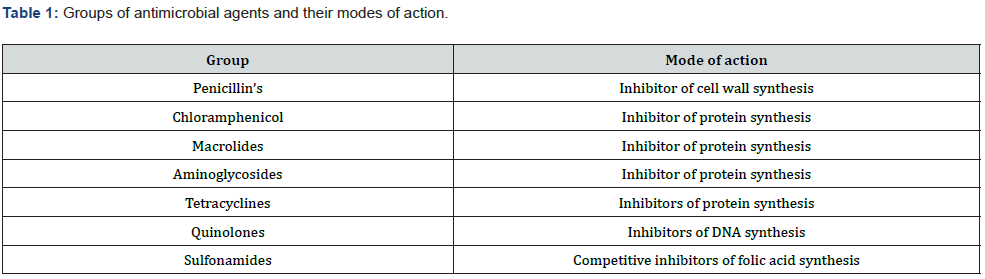
Mechanism of Action: To appreciate the mechanisms of resistance, it is important to understand how antibacterial agents act. The mechanism of action is the biochemical way in which a drug is pharmacologically effective (Table 1). This can be a specific target where the drug binds like an enzyme, as is the case with many antibiotics, or a receptor. Mechanism of action describes the biochemical process specifically at a molecular level. One of the most common mechanisms of action is targeting the cell wall, which is present in bacteria (prokaryotic cells) but absent in humans (eukaryotic cells). Thus, antimicrobial agents act selectively on vital microbial functions with minimal effects or without affecting host functions. Different classes of antibiotics possess specific modes of action by which they inhibit the growth or kill bacteria (Figure 2) Ali [16].
I. Inhibitor of Cell Wall Synthesis
Bacterial cells are surrounded by a cell wall made of peptidoglycan, which consists of long sugar polymers. The peptidoglycan undergoes cross-linking of the glycan strands by the action of trans glycosidases, and the peptide chains extend from the sugars in the polymers and form cross links, one peptide to another. The D-alanyl-alanine portion of peptide chain is cross linked by glycine residues in the presence of penicillin binding proteins (PBPs). This cross-linking strengthens the cell wall. The β-lactams and the glycopeptides inhibit cell wall synthesis Reynolds [17].
The primary targets of the β-lactam agents are the PBPs. It has been hypothesized that the β-lactam ring mimics the D-alanylalanine portion of peptide chain that is normally bound by PBP. The PBP interacts with β-lactam ring and is not available for the synthesis of new peptidoglycan. The disruption of peptidoglycan layer leads to the lysis of bacterium Džidic [18]. The glycopeptides bind to D-alanyl -alanine portion of peptide side chain of the precursor peptidoglycan subunit. The large drug molecule vancomycin prevents binding of this D-alanyl subunit with the PBP, and hence inhibits cell wall synthesis Džidic [18].
Source: Ali [16]
II. Inhibitors of Protein Biosynthesis
First the information in bacterial DNA is used to synthesize an RNA molecule referred to as messenger RNA (m-RNA) a process known as transcription. Then, the macromolecular structure called ribosome synthesizes proteins present in m-RNA, a process called translation. Protein biosynthesis is catalyzed by ribosomes and cytoplasmic factors. The bacterial 70S ribosome is composed of two ribonucleoprotein subunits, the 30S and 50S subunits. Antibiotics inhibit protein biosynthesis by targeting the 30S or 50S subunit of the bacterial ribosome Johnston & Vannuffel, Cocito [19,20].
III. Folic Acid Metabolism Inhibitors
Folic acid is an essential nutrient necessary for protein and nucleic acid synthesis (DNA and RNA). It is a powerful freeradical scavenger that provides methyl donors; it is a watersoluble vitamin (B9). Folic acid is synthesized by bacteria from the substrate, para-amino-benzoic acid (PABA), and all cells require folic acid for growth. Folic acid (as a vitamin in food) diffuses or is transported into mammalian cells. However, folic acid cannot cross bacterial cell walls by diffusion or active transport. For this reason, bacteria must synthesize folic acid from PABA. Sulfonamides act by competing with PABA as a substrate for the enzyme dihydropteroate synthase, which incorporates PABA into dihydropteroic acid, the immediate precursor of folic acid. Each of these drugs inhibits distinct steps in folic acid metabolism. A combination of sulpha drugs and trimethoprim acting at distinct steps on the same biosynthetic pathway shows synergy and a reduced mutation rate for resistance. Sulfonamides inhibit dihydropteroate synthase in a competitive manner with higher affinity for the enzyme than the natural substrate, p-amino benzoic acid. Agents such as trimethoprim act at a later stage of folic acid synthesis and inhibit the enzyme dihydrofolate reductase Yoneyama & Katsumata [21].
Antibiotic Resistance
Antimicrobial resistance (AMR) is the ability of a microbe to resist the effects of medication that once could successfully treat the microbe. The term antibiotic resistance (ABR) is a subset of AMR, as it applies only to bacteria becoming resistant to antibiotics. Antibiotic resistance is the ability of a bacterium to survive and reproduce in the presence of antibiotic doses that were previously thought effective against them. The origin of antibiotic resistance genes is unclear; however, studies using clinical isolates collected before the introduction of antibiotics demonstrated susceptibility, although conjugative plasmids were present. Resistant microbes are more difficult to treat, requiring alternative medications or higher doses of antimicrobials. These approaches may be more expensive, more toxic or both Dubois [7]. Several of various recent studies revealed that almost 400 different bacteria have demonstrated about 20,000 possible resistant genes Davies & Davies [8].
Normally, most cells in a naive, susceptible bacterial population which can cause an infection are susceptible to antibiotics upon exposure. However, there is always a minute subpopulation of resistant bacterial cells that will be able to multiply at higher concentrations in insufficient antibiotic concentration which kill the subpopulation so that micro-organisms survive in the environment. Resistance is often associated with reduced bacterial fitness, and it has been proposed that a reduction in antibiotic use will pose selective pressure to acquire resistance would benefit the fitter susceptible bacteria, enabling them to outcompete resistant strains over time Smith [22].
Antibiotic resistant bacteria are a growing public health emergency since infections from resistant bacteria are harder and costly to treat. For instance, since the 1990s, some strains of Salmonella became resistant to a range of antibiotics. Resistance is supposed to occur from the use of antibiotics in human and animal husbandry. The major problem in clinical practice today is the emergence of multiple-drug resistance, which is resistance to several types of antimicrobial agent Amenu [23].
Veterinary Source of Antibiotic Resistance
Bacteria with resistance to antibiotics predate medical use of antibiotics by humans and animals. However, widespread antibiotic use has made more bacteria resistant through the process of evolutionary pressure. Reasons for the widespread use of antibiotics in human and animal medicine include: increasing global availability over time since the 1950s, uncontrolled sale in many low or middle income countries, where they can be obtained over the counter without a prescription, antibiotic use in livestock feed at low doses for growth promotion is an accepted practice in many industrialized countries and is known to lead to increased levels of resistance, releasing large quantities of antibiotics into the environment during pharmaceutical manufacturing through inadequate wastewater treatment increases the risk that antibiotic-resistant strains will develop and spread. It is uncertain whether antibacterial in soaps and other products contribute to antibiotic resistance Bennett [24].
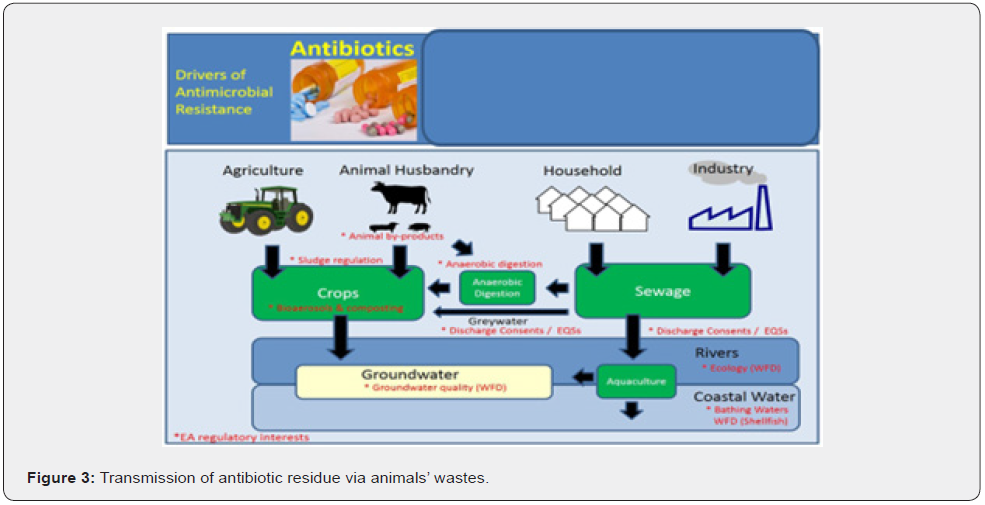
Animal Wastes: Antibiotics are not fully digested and processed in the animal; therefore, an estimated 40 to 90% of the antibiotics ingested and administered are excreted in the animal’s urine and/or feces. The presence of antibiotics in animal waste has been widely reported worldwide from multiple farms of different species. The high amounts of antibiotics present in animal waste, even after long periods of composting, are a potential pollution source of environmental antibiotics. Animal waste is often recycled and used as fertilizer for crops (Figure 3). Consequently, antibiotics, while small amounts, have been found in crops grown in these fertilized fields. In another study, antibiotics were detected in runoff from animal waste fertilized lands at concentrations up to 9 Nano-grams per liter. In some cases, animal waste is directly released to receiving watersheds through intentional discharge or leaching from farms. Any person who has access to those sources exposed to antibiotic residue which causes antibiotic resistance FWW [25].
Animal Source Foods: Antibiotics that are used in humans to treat or prevent certain diseases either belong to the same general class or have the same mode of action as those used for animals Joshi [3]. These drugs, despite their effectiveness, can leave residues in the treated animal and contaminate its edible parts; the muscle meat and milk Zeina [5]. The residues occur mostly when animals are slaughtered and milked within the withdrawal period of the drug. The presence of residues of antibiotics in beef and milk has a great public health concern. Human consumption of these may be associated with the development of antibiotic resistance in human pathogens that are difficult to treat with the commonly available antibiotics Alla [6]. They range from direct toxicity on consumers exhibiting allergic reactions to indirect problems through the generation of resistant strains of pathogenic bacteria and the residual contamination of manures used in crop productions. They result in adverse public health effects including hypersensitivity, tissue damage, gastrointestinal disturbance, and bacterial resistant strain Dubois [7].
Antibiotic residues can accumulate in egg components when administered to laying hens or when laying hens are mistakenly given medicated feed, or when the diet of egg-laying strains of chickens is accidently contaminated at the feed mill due to prior use of antibiotics with other feeds. The traces of antimicrobial drugs in the eggs of hens are an important public health issue European Commission [26]. Antibiotic residues can also occur in fish flesh when the drugs are administered outside the labeled dose or recommendations. Extra-label usage of antibiotics in aquaculture is practiced. Chloramphenicol, dimetridazole, ipronidazole, nitroimidazoles, furazolidone, nitrofurazone, and fluoroquinolones are prohibited for extra-label use in foodproducing animals FDA [27].
Animal feed additives: Many antibiotics that are used in animal feed are also used to treat diseases in man. Such use of antibiotics in feed raised concern among public health authorities and consumers because such level use of the drug may cause occurrence of bacterial resistance in the gastrointestinal tract (GIT) of these animals. Such resistance can also transfer to bacterial inhabitants of the GIT through food chain to the public Marshall & Levy [28]. The feeding of low levels of antibiotics such as tetracycline and penicillin in poultry, swine, and calves to promote growth has resulted in a great increase in the reservoir of resistant bacteria. These resistant bacteria from animals may reach the human population. Antibiotic resistant bacteria spread from animals to human indirectly via food (e.g., by contamination of carcasses during slaughter), or less commonly by direct contact (farmers, abattoir workers) Dugassa [29].
Water pollution: Antibiotic resistance is a growing problem among humans and wildlife in terrestrial or aquatic environments. In this respect, the spread and contamination of the environment, especially through water pollution (“hot spots”) is a growing and serious public health problem Levy & Marti [30,31]. Antibiotics have been polluting the environment since their introduction through animal wastes (medication, farming) and the pharmaceutical industry. The contribution of the pharmaceutical industry is so significant that parallels can be drawn between countries with the highest rate of increasing antibiotic resistance and countries with largest footprint of pharmaceutical industry. China, which contributes to nearly 90 per cent of the world’s active pharmaceutical ingredient (API) manufacturing, has seen a 22 per cent increase in rate of antimicrobial resistance in six years, compared to a 6 per cent increase in the United States Marti [31].
Along with antibiotic waste, resistant bacteria follow, thus introducing antibiotic-resistant bacteria into the environment. As bacteria replicate quickly, the resistant bacteria that enter water bodies through wastewater replicate their resistance genes as they continue to divide. In addition, bacteria carrying resistance genes can spread those genes to other species via horizontal gene transfer. Therefore, even if the specific antibiotic is no longer introduced into the environment, antibioticresistance genes will persist through the bacteria that have since replicated without continuous exposure Marti [31]. Antibiotic resistance is widespread in marine vertebrates, and they may be important reservoirs of antibiotic-resistant bacteria in the marine environment Rose [32].
Sub-therapeutics: The use of antibiotics at recommended dosage levels to treat confirmed bacterial infections is a type of exposure for which the benefit far outweighs the risk of selecting resistant strains Rafailidis [33]. Unfortunately, much of the antibiotic therapy in livestock and fishery is not body weight oriented. This coupled with the high proportion of lifethreatening infections that require immediate treatment. The use of sub-therapeutic doses (mainly by improper prescription or patient non-compliance) which creates a situation whereby highly resistant strains are selected sequentially Moran [34].
Inappropriate prescription: Inappropriate prescribing of antibiotics has been attributed to several causes, such as patients insisting on antibiotics and physicians prescribing them as they do not have time to explain why they are not necessary. Another cause can be physicians not knowing when to prescribe antibiotics or being overly cautious for medical or legal reasons. For example, most cases of diarrhea in claves are caused by viral pathogens, for which antibiotics are not effective. But nevertheless, around 40 percent of these cases are attempted to be treated with antibiotics. In some areas even over 80 percent of such cases are attempted to be treated with antibiotics. Such treatment causes antibiotic resistance of GIT microbes of animal that pose high public health impact Araya [35].
Cost of antibiotics: The treatment of infected animals in many parts of Africa is further challenged by the fact that prohibitive cost of newer second-line antibiotics like amoxicillin-clavulanate, cefuroxime, ceftriaxone, ofloxacin, ciprofloxacin, azithromycin, amikacin, and others, when available, places them out of the reach for most patients. Since there is no broad enough selection profile of the second-line drugs, there is usually no cost-effective customization of empiric antibiotic therapy. Customer prefers low costs antibiotics, and these low-cost antibiotics are poor quality that leads development of antibiotic resistance Silbergeld [36].
Poor storage: The other factor is the poor storage of antibiotics which leads to drug degradation by heat and/or humidity during distribution. In addition, overcrowding and lack of resources for effective storage of antibiotics in many veterinary clinics, pharmacies, and importers. Poor storage condition and inappropriate distribution decrease the effectiveness of antibiotics. Administration of poor-quality antibiotics increase epidemics of resistant organisms such as methicillin resistant staphylococci aureus and others Kieninger & Lipsett [37].
Natural occurrence: Naturally occurring antibiotic resistance is common. Genes for resistance to antibiotics are ancient D’Costa1 [38]. The genes that confer resistance are known as the environmental resistome. These genes may be transferred from non-disease-causing bacteria to those that do cause disease, leading to clinically significant antibiotic resistance Wright [39].
In 1952 it was shown that penicillin-resistant bacteria existed before penicillin treatment, and preexistent bacterial resistance to streptomycin. In 1962, the presence of penicillinase was detected in dormant endospores of Bacillus licheniformis, revived from dried soil on the roots of plants, preserved since 1689 in the British Museum. Six strains of Clostridium, found in the bowels of William Braine and John Hartnell (members of the Franklin Expedition) showed resistance to cefoxitin and clindamycin Davies & Davies [8].
Mechanism of Antibiotic Resistance
As there are many ways in which antibiotics can kill or inhibit the growth and multiplication of bacteria, there are also many mechanisms of resistance that microorganisms innately possess or have developed over time through exposure of antibiotics. It is possible that through one mechanism, an organism can become resistant to many different classes of antibiotics, especially if the modes of action are similar. A bacterium is resistant if it exhibits significantly reduced susceptibility when compared with that of the original isolate or a group of sensitive strains. Resistance can result from mutations in housekeeping structural or regulatory genes, or alternatively, horizontal acquisition of foreign genetic information Courvalin [40]. Sometimes resistance can be shared between individual bacteria through the production of resistance plasmids, the pieces of DNA capable of being transferred from one cell to another Clewell [41].
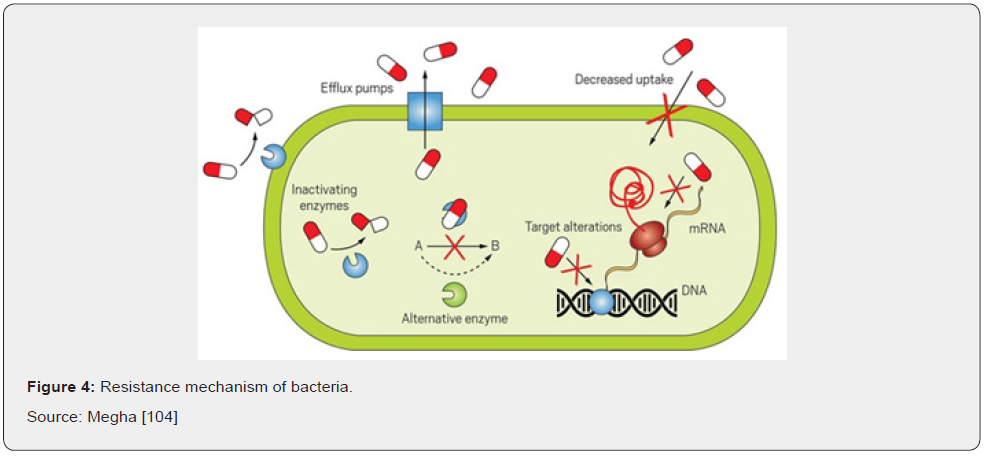
Resistance to an antibiotic may be an inherent property of the infecting organism or it may be acquired. Acquired resistance may result from mutation or from transfer of an extra chromosomal genetic material followed by selection of resistant organisms during therapy Courvalin [40]. There is a range of mechanisms by which an organism can acquire resistance, the simplest being genetic mutation. Resistant mutants will have a strong survival advantage in the face of antibiotic exposures, giving rise to the total usage of antibacterial agents in a population and the increased proportion of isolates that exhibit resistance to those agents Hegstad [42] (Figure 4).
Intrinsic or natural: In this case bacteria naturally do not possess target sites for the drugs and therefore the drug does not affect them, or they naturally have low permeability to those agents because of the differences in the chemical nature of the drug and the microbial membrane structures especially for those that require entry into the microbial cell to affect their action. An example of natural resistance is Pseudomonas aeruginosa, whose low membrane permeability is likely to be a main reason for its innate resistance to many antibiotics. Other examples are the presence of genes affording resistance to self-produced antibiotics, the outer membrane of Gram-negative bacteria, absence of an uptake transport system for the antibiotics or general absence of the target or reaction hit by the antibiotics Yoneyama & Katsumata [21].
Acquired or active resistance: The major mechanism of antibiotic resistance is the result of a specific evolutionary pressure to develop a counterattack mechanism against an antibiotic or class of antibiotic so that bacterial populations previously sensitive to antibiotics become resistant. This type of resistance results from changes in the bacterial genome. Resistance in bacteria may be acquired by a mutation and passed vertically by selection to daughter cells. More commonly, resistance is acquired by horizontal transfer of resistance genes between strains and species. Exchange of genes is possible by transformation, transduction, or conjugation Langton [43]. Acquired resistance mechanisms can occur through various ways.
I. Antibiotic inactivation
On some occasions cells may gain resistance to antibiotics by making an enzyme that renders the drug inactive, or that decreases the functionality of the antibiotics. There are three main enzymes that inactivate antibiotics such as β-lactamases, aminoglycosidemodifying enzymes, and chloramphenicol acetyltransferases. The best example is beta lactamases that have ester and amide bond. Genes encoding for β-lactamases are generally termed bla, followed by the name of the specific enzyme (e.g., blaKPC) and they have been found in the chromosome or localized in mobile gene element (MGEs) as part of the accessory genome. In terms of their expression, transcription of these genes can be constitutive, or it may require an external signal to induce their production. The enzyme β-lactamases has capable of breaking the beta-lactam rings of beta lactam antibiotics such as penicillin, cephalosporins, monobactams, and carbapenems. In such manner, the breakage of the beta-lactam ring stops the antibiotic from being able to attach to the peptidoglycan precursors. But it will be less likely that penicillin or other similar drugs will be able to disrupt the integrity of the cell wall if the organism produces beta lactamases Sageman[44]. This method of resistance can be transferred from one bacterium to another through the production of plasmids and is common in strains of methicillin resistant Staphylococcus aureus (MRSA) Holcomb [45].
II. Reduced membrane permeability.
Many of the antibiotics used in clinical practice have intracellular bacterial targets or, in case of gram-negative bacteria, located in the cytoplasmic membrane (the inner membrane). Therefore, the compound must penetrate the outer and/or cytoplasmic membrane to exert its antibiotics effect. Bacteria have developed mechanisms to prevent the antibiotic from reaching its intracellular or periplasmic target by decreasing the uptake of the antibiotic’s molecule. This mechanism is particularly important in gram-negative bacteria, limiting the influx of substances from the external milieu. In fact, the outer membrane acts as the first line of defense against the penetration of multiple toxic compounds, including several antibiotics agents. Hydrophilic molecules such as β-lactams, tetracyclines and some fluoroquinolones are particularly affected by changes in permeability of the outer membrane since they often use water-filled diffusion channels known as porins to cross this barrier. The prime example of the efficiency of this natural barrier is the fact that vancomycin, a glycopeptide antibiotic, is not active against gram-negative organisms due to the lack of penetration through the outer membrane. Likewise, the innate low susceptibility of Pseudomonas and Acinetobacter baumanii to β-lactams (compared to Enterobacteriaceae) can be explained, at least in part, to a reduced number and/or differential expression of porins Hancock & Brinkman [46].
Several types of porins have been described, and they can be classified according to their structure, their selectivity, and the regulation of their expression. Among the best-characterized porins, the three major proteins produced by E. coli (known as OmpF, OmpC and PhoE) and the P. aeruginosa OprD (also known as protein D2) are classical examples of porin-mediated antibiotic resistance. Alterations of porins could be achieved by 3 general processes, i) a shift in the type of porins expressed, ii) a change in the level of porin expression, and iii) impairment of the porin function. Importantly, changes in permeability through any of these mechanisms frequently result in low-level resistance and are often associated with other mechanisms of resistance, such as increased expression of efflux pumps Nikaido [47].
III. Chemical alterations of the antibiotic
The production of enzymes capable of introducing chemical changes to the antibiotic’s molecule is a well-known mechanism of acquired antibiotic resistance in both gram negative and grampositive bacteria. Interestingly, most of the antibiotics affected by these enzymatic modifications exert their mechanism of action by inhibiting protein synthesis at the ribosome level. Many types of modifying enzymes have been described, and the most frequent biochemical reactions they catalyze include i) acetylation (aminoglycosides, chloramphenicol, streptogramins), ii) phosphorylation (aminoglycosides, chloramphenicol), and iii) adenylation (aminoglycosides, lincosamides). Regardless of the biochemical reaction, the resulting effect is often related to steric hindrance that decreases the avidity of the drug for its target Wilson [48].
One of the best examples of resistance via modification of the drug is the presence of aminoglycoside modifying enzymes (AMEs) that covalently modify the hydroxyl or amino groups of the aminoglycoside molecule. These enzymes are usually harbored in mobile genetic elements (MGEs), but genes coding for AMEs have also been found as part of the chromosome in certain bacterial species Ramirez and Tolmasky [49]. Another classical example of enzymatic alteration of an antibiotic involves the modification of chloramphenicol, an antibiotic that inhibits protein synthesis by interacting with the peptidyl-transfer center of the 50S ribosomal subunit. The chemical modification of chloramphenicol is mainly driven by the expression of acetyltransferases known as CATs (chloramphenicol acetyltransferases). Multiple cat genes have been described in both gram-positives and gram-negatives and they have been classified in two main types. Type A, which usually results in high-level resistance, and type B that confers low-level chloramphenicol resistance Schwarz [50].
IV. Changes in target sites
Many antibiotics act by binding to a target molecular component of the bacteria. Natural variations or acquired changes in the target sites of antibiotics that prevent drug binding are a common mechanism of resistance. Target site changes often result from spontaneous mutation of a bacterial gene on the chromosome. Since antibiotic interaction with target molecule is generally quite specific, minor alteration of the target molecule can have an important effect on antibiotic binding. Bacteria can decrease the effectiveness of antibiotics if the target molecule changes slightly in its structure so that antibiotics may no longer be able to bind to the target molecule. For example, tetracyclines block the transfer RNA access site by binding to it. In turn slight changes in the access site may result in microbial resistance to tetracyclines Dubois [7].
Target sites protection is one of the resistance methods of bacteria for antibiotics. Although some of the genetic determinant’s coding for proteins that mediate target protection have been found in the bacterial chromosome, most of the clinically relevant genes involved in this mechanism of resistance are carried by mobile gene elements. Target sits protection of bacteria by protein-protein interaction, wherein an antibiotic resistance protein directly binds the drug target protein and acts to protect it from the inhibitory effects of an antibiotic. Examples of drugs affected by this mechanism include tetracycline (Tet[M] and Tet[O]), fluoroquinolones (Qnr) and fusidic acid (FusB and FusC). One of the classic and best-studied examples of the target protection mechanism is the tetracycline resistance determinants Tet(M) and Tet(O). TetO and TetM interact with the ribosome and dislodge the tetracycline from its binding site in a GTP-dependent manner Jose & Cesar [51].
Mutations of the target site are another method of resistance. One of the most classical examples of mutational resistance involves the mechanism of FQ resistance. FQs kill bacteria by altering DNA replication through the inhibition of two crucial enzymes, DNA gyrase and topoisomerase IV. Development of chromosomal mutations in the genes encoding subunits of the above-mentioned enzymes (gyrA-gyrB and parC-parE for DNA gyrase and topoisomerase IV, respectively) is the most frequent mechanism of acquired resistance to these compounds Jose & Cesar [51].
Enzymatic alteration of the target site: One of the best characterized examples of resistance through enzymatic modification of the target site is the methylation of the ribosome catalyzed by an enzyme encoded by the erm genes (erythromycin ribosomal methylation), which results in macrolide resistance. These enzymes are capable of mono- or di-methylating an adenine residue in position A2058 of the domain V of the 23rRNA of the 50S ribosomal subunit. Due to this biochemical change, the binding of the antibiotics molecule to its target is impaired. Importantly, since macrolides, lincosamides, and streptogramin B antibiotics have overlapping binding sites in the 23S rRNA, expression of the erm genes confers cross-resistance to all members of the MLSB group Tenover [52].
Complete replacement or bypass of the target site is the other strategy that bacteria can evolve new targets that accomplish similar biochemical functions of the original target but are not inhibited by the antibiotic’s molecule. The most relevant clinical examples include methicillin resistance in S. aureus due to the acquisition of an exogenous PBP (PBP2a) and vancomycin resistance in enterococci through modifications of the peptidoglycan structure mediated by the van gene clusters Grundmann [53].
Alteration in penicillin binding protein (PBP) is a favored mechanism of resistance to Gram-positive bacteria. The presence of mutation in penicillin-binding protein leads to a reduced affinity to β-lactam antibiotics. The resistance of Enterococcus faecium to ampicillin and Streptococcus pneumoniae to penicillin is by this mechanism. Similarly, in Staphylococcus aureus, the resistance to methicillin and oxacillin is associated with integration of a mobile genetic element “staphylococcal cassette chromosome mec” into the chromosome of S. aureus that contains resistance gene mec A. mec A gene encodes PBP2a protein, a new penicillin-binding protein, that is required to change a native staphylococcal PBP. PBP2a shows a high resistance to β-lactam antibiotics. S. aureus strains resistant to methicillin can be cross resistant to all β-lactam antibiotics, streptomycin, and tetracycline and in some cases to erythromycin Grundmann [53].
The other methods of binding site modifications are altered cell wall precursors. Cell wall synthesis in Gram-positive bacteria can be inhibited by glycopeptides, e.g., vancomycin or teicoplanin, by their binding to D-alanyl-D-alanine residues of peptidoglycan precursors. D-alanyl-alanine is changed to D-alanyl-lactate because of which glycopeptides do not cross link with them, hence resistance to them develops. E. faecium and E. faecalis strains have high resistance to vancomycin and teicoplanin (Van A-type resistance) Grundmann [53]. Quinolones bind to DNA gyrase A subunit. The mechanism of resistance involves the modification of two enzymes: DNA gyrase (coded by genes gyr A and gyr B) and topoisomerase IV (coded by genes par C and par E). Mutations in genes gyr A and par C lead to replication failure and as a result FQ cannot bind Kim [54].
V. Efflux or transport of antibiotic
The production of complex bacterial machinery capable of extruding a toxic compound out of the cell can also result in antibiotic resistance. This is another mechanism by which bacteria can become resistant to antibiotics by utilizing an efflux pump. An efflux pump is a biological pump that can force the antibiotic out of the cell, so that it cannot reach or stay in contact with its target (Figure 5). This method of antibiotic resistance may often create resistance to more than one class of antibiotics, especially the macrolides, tetracyclines, and fluoroquinolones because these antibiotics inhibit different aspects of protein and DNA biosynthesis and therefore must be intracellular to exert their effect Willey [55].
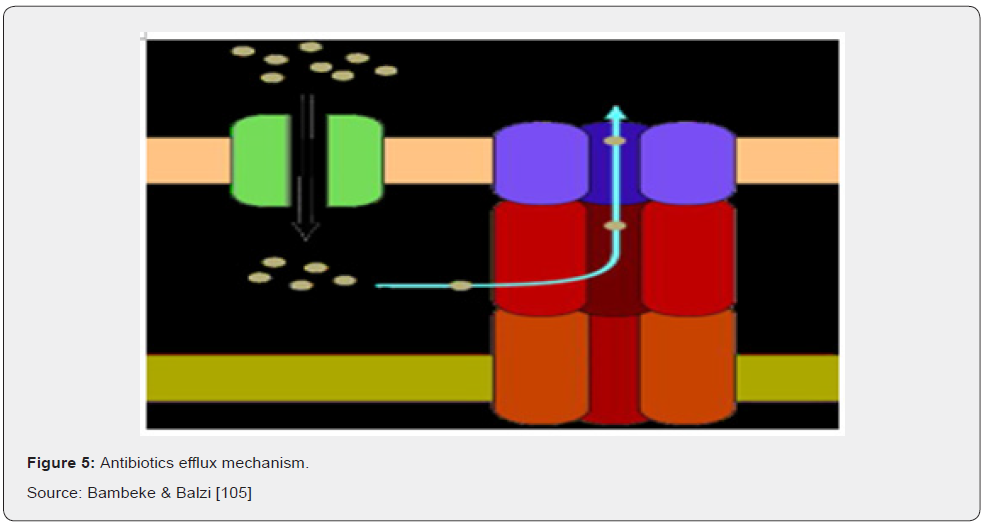
There are 5 major families of efflux pumps, including i) the major facilitator superfamily (MFS), ii) the small multidrug resistance family (SMR), iii) the resistance-nodulation-celldivision family (RND), iv) the ATP-binding cassette family (ABC), and v) the multidrug and toxic compound extrusion family (MATE). These families differ in terms of structural conformation, energy source, range of substrates they can extrude and in the type of bacterial organisms in which they are distributed. Tetracycline resistance is one of the classic examples of effluxmediated resistance, where the Tet efflux pumps (belonging to the MFS family) extrude tetracyclines using proton exchange as the source of energy Ali [16].
V.5 Multiple Antibiotic Resistances
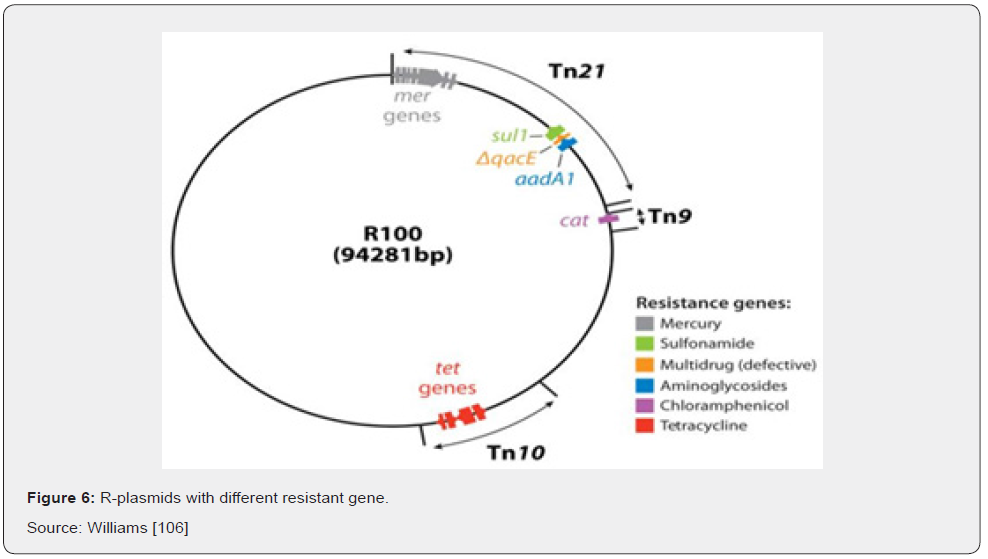
R-plasmids possess regions with resistance genes and resistance to several different antibiotics that can be mediated by the same R-factor and are known as multiple antibiotic resistances (Figure 6) Ali [16]. The prevalence of multiple drug resistance bacteria itself is a serious problem but transfer of multiple drug resistance to other members of the family Enterobacteriaceae, particularly E. coli, Salmonella and Shigella makes it even greater concern to clinicians in curbing infections in medical and veterinary practice Ali [16].
V.6 Transfer of Antibiotic Resistance Genes
Any gene has the potential to be transferred between bacteria, including antibiotic resistance genes. Whether or not transferred genes will be integrated into the DNA of a recipient bacterium is another question. Foreign DNA can be harmful to a bacterium, and there are machineries in place that degrades incoming DNA. However, these systems are not 100% efficient. If the incoming DNA is incorporated and provides a benefit for the bacterium it is more likely maintained. For example, if a bacterium picks up an antibiotic resistance gene and is subsequently exposed to that antibiotic, this bacterium will be better off than susceptible neighbors and can increase in number. Bacteria can share genes with each other in a process called horizontal gene transfer. This can occur both between bacteria of the same species and between different species and by several different mechanisms, given the right conditions. Gene transfer results in genetic variation in bacteria and is a large problem when it comes to the spread of antibiotic resistance genes Bbosa [56].
V.6.1 Horizontal gene transfer: Horizontal gene transfer is genetic modification by microorganisms themselves and is a very efficient and rapid way of transferring resistance between populations. It is the most relevant mode of resistance emergence and spread in microbial populations. Acquisition of foreign DNA material through HGT is one of the most important drivers of bacterial evolution and it is frequently responsible for the development of antibiotics resistance to Bbosa [56]. The main methods are transformation, transduction, and conjugation.
I. Transformation
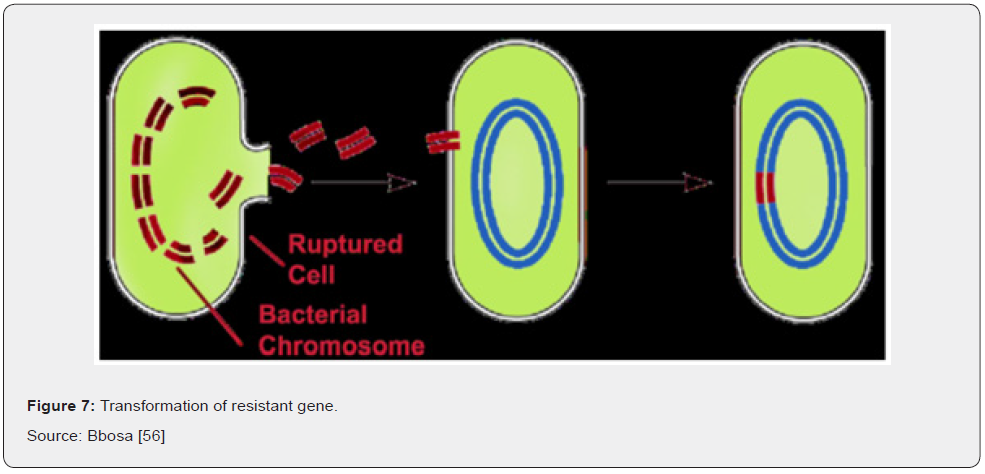
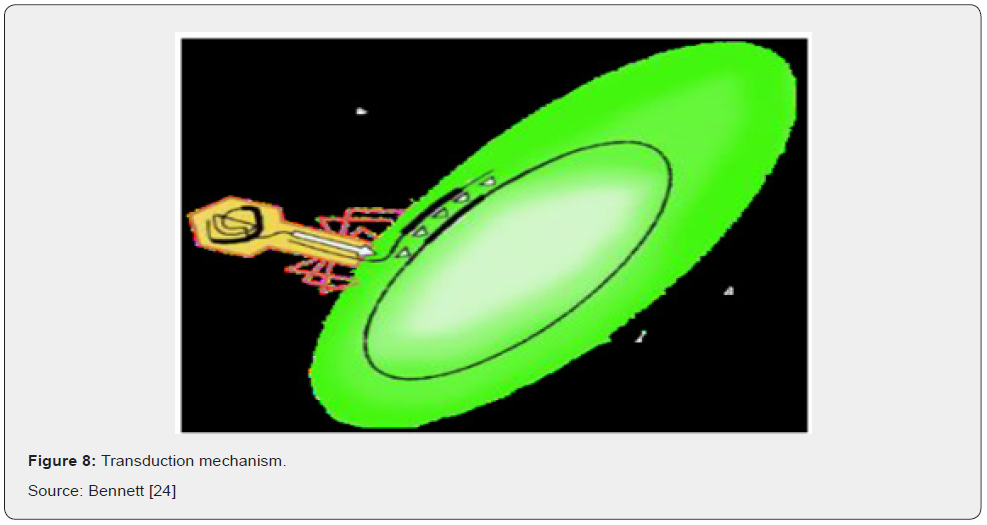
Transformation refers to the ability of microorganisms to utilize snippets of free DNA from their surroundings. DNA from dead cells gets cut into fragments and exits the cell (Figure 7) Bbosa [56]. Transduction is the process by which viruses that prey upon bacteria, known as bacteriophages, can transmit genetic material from one organism to another. It is like the way mosquitos transmit disease from animal to animal. However, while the mosquito is a passive carrier, bacteriophages are more complicated. Being viruses themselves they inject their genetic material into a bacterial cell and replicate there to a great degree. Their normal mode of reproduction is to harness the replicational, transcriptional, and translation machinery of the host bacterial cell to make numerous virions, or complete viral particles, including the viral DNA or RNA and the protein coat Bbosa [56]. The packaging of bacteriophage DNA has low fidelity and small pieces of bacterial DNA, together with the bacteriophage genome, may become packaged into the bacteriophage genome. At the same time, some phage genes are left behind in the bacterial chromosome. When the cell eventually ruptures it emits many more bacteriophages into the surroundings to infect other microorganisms Bennett [24] (Figure 8).
II. Conjugation
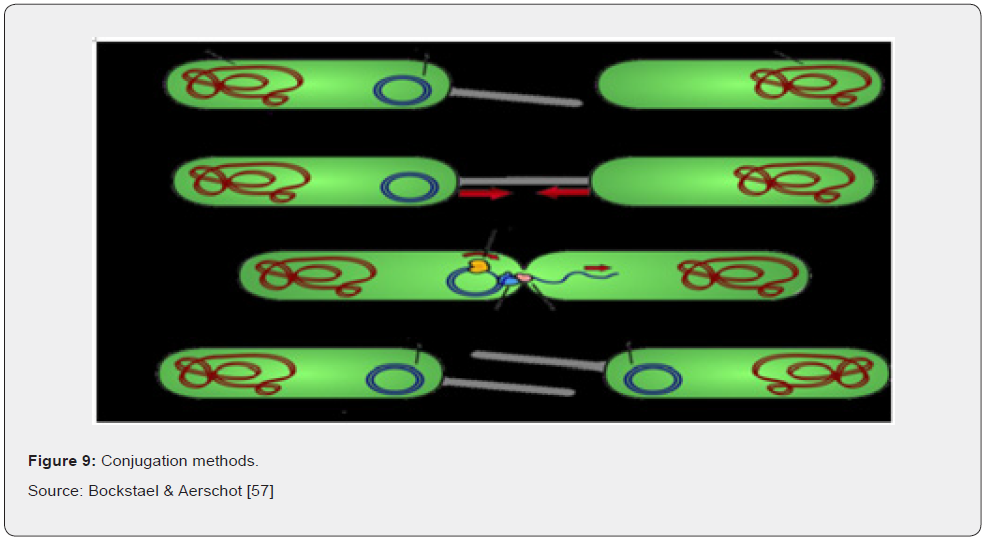
Bacterial conjugation is the transfer of genetic material between bacterial cells by direct cell-to-cell contact or by a bridgelike connection between two cells. It is a mechanism of horizontal gene transfer as are transformation and transduction although these two other mechanisms do not involve cell-to-cell contact. Bacterial conjugation is often regarded as the bacterial equivalent of sexual reproduction or mating since it involves the exchange of genetic material. During conjugation the donor cell provides a conjugative or mobilizable genetic element that is most often a plasmid or transposon (Figure 9). The fact that this process can occur easily between different species of bacteria makes Bockstael & Aerschot [57].
V.7 Economic Significance of Antibiotic Resistance
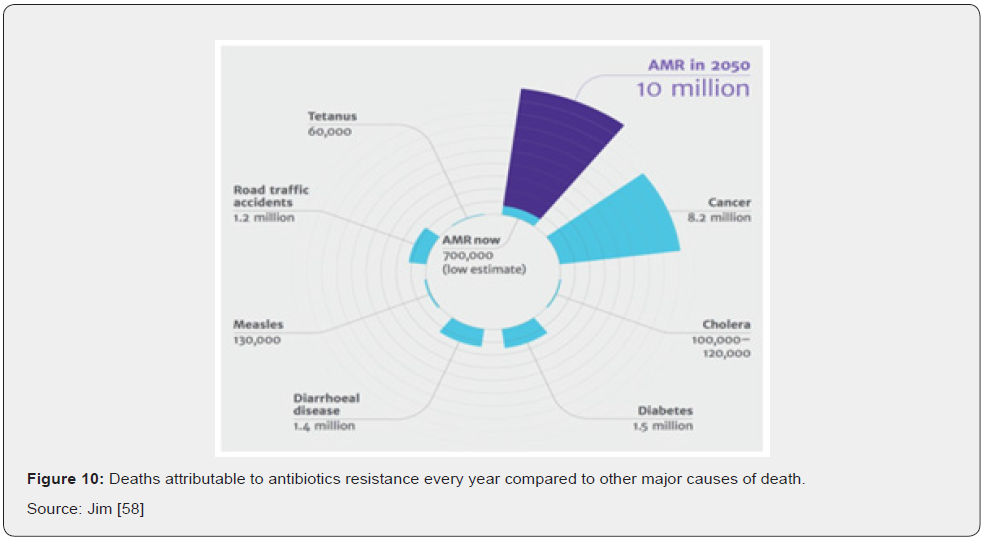
The detrimental health effects produced by antibiotic resistance go hand in hand with a negative impact on the budget of health systems and, more broadly, on the economy. From the micro-level to the macro-level, antibiotic resistant microorganisms have a direct negative impact on many actors and economic dimensions. First, by requiring more intensive therapies, antibiotic resistance increases health expenditures. Second, patients and their families may undergo additional non-healthcare related expenditures (e.g., travel time) or suffer from income loss due to ill health and dearh (Figure 10). At the societal level, antibiotic resistance negatively impacts labor market outcomes due to absence from work which, down the line, negatively affects the broader economic performances of countries Jim [58]. Costs for laboratory tests, radiologic studies, bronchoscopies, or other diagnostic procedures are part of diagnostic costs and primarily of concern to the health-care institution when these costs cannot be passed on to the patient or an insurer. The same is true of costs for purchase and administration of antibiotics drugs and other therapeutic agents. Patients experience both direct costs of health care and indirect costs (e.g., loss of productivity resulting in a reduction in income). Other types of indirect costs of antibiotics drug resistance are costs to the drug industry resulting from diminishing marketability of their drugs and costs to businesses for loss of workers’ productive time. All these factors are part of the economic impact of resistance Jim [58] (Figure 11).
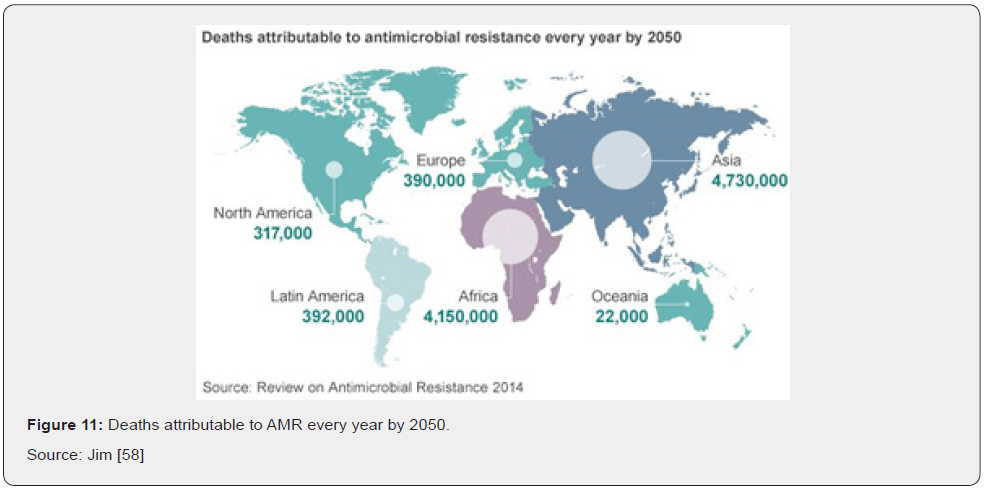
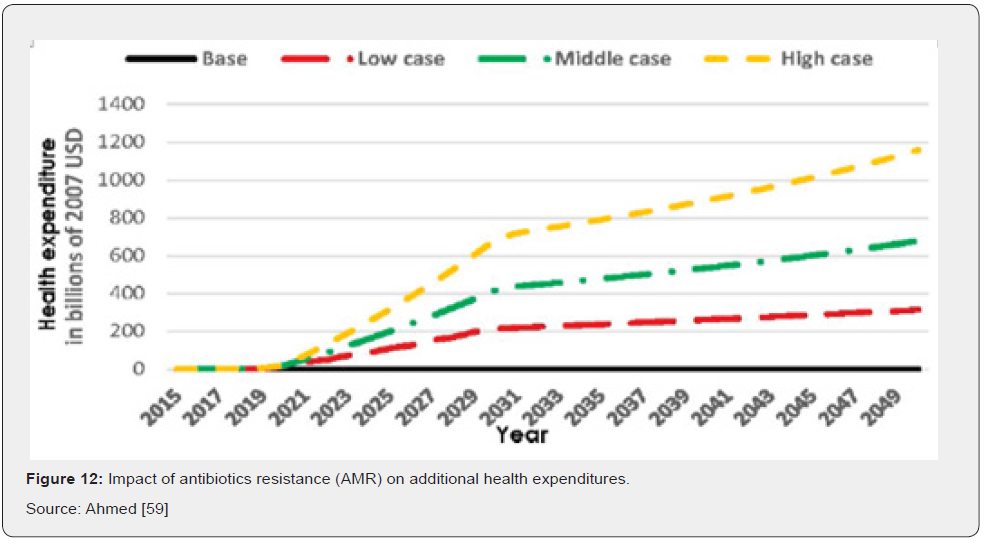
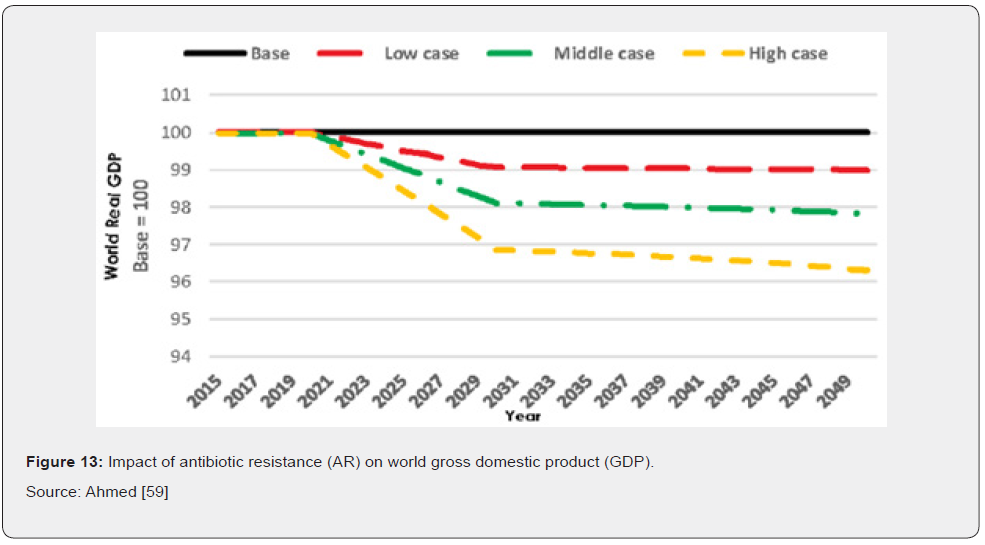
In terms of direct costs of antibiotic resistance, health care expenditures would increase in tandem with rising cases of AR. Results also show that additional health care expenses in 2050 would be $0.33 trillion in the low AR scenario, while in the high AR scenario they would amount to $1.2 trillion annually (Figure 12) Ahmed [59]. For indirect costs AR has a significant negative impact on GDP. Compared to the base case, GDP would be 1.1 percent lower in 2050 under the low AR scenario and 3.8 percent lower in 2050 under the high AR scenario. Similar effects of decline are seen regarding global livestock exports (Figure 13) Ahmed [59].
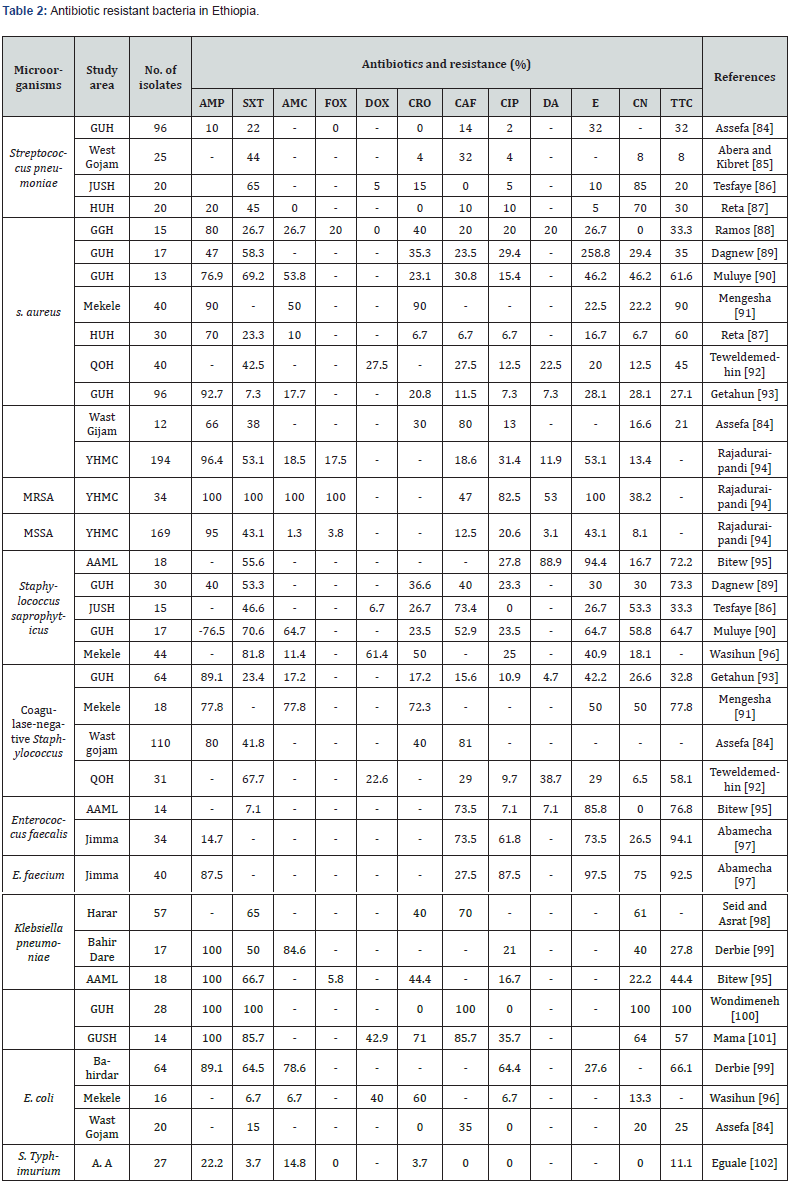
AMP: Ampicillin; SXT: Trimethoprim-Sulfamethoxazole; AMC: Amoxicillin-Clavulanic acid; Dox: Doxycycline; CRO: Cefriaxone; CAF: Chloramphenicol; CIP: Ciprofoxacin; DA: Clindamycin; E: Erythromycin; CN: Gentamicin; TTC: Tetracycline; FOX: Cefoxitin; GUH: Gonder University Hospital; JUSH: Jimma University Specialized Hospital; HUH: Hawassa University Hospital; YHMC: Yekatit 12 Hospital Medical College; QOH: Quiha Ophthalmic Hospital; AAML: Arsho Advanced Medical laboratory; MRSA: Methicillin-Resistant Staphylococcus aureus; MSSA: Methicillin-Sensitive Staphylococcus aureus.
Recent Drug Target on DNA Replication
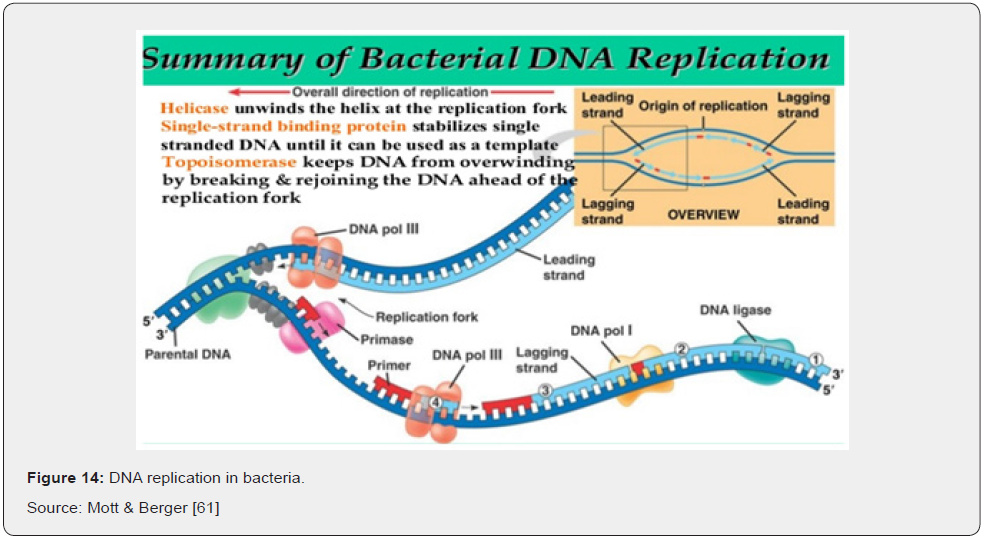
Most bacteria contain a single circular chromosome, within which replication is initiated at a single site, the origin of replication, oriC. The two strands of the template DNA are separated at the origin, yielding two fork structures. Replicative DNA polymerases (replicases) and accessory proteins are assembled onto each of these forks and synthesize new DNA bi-directionally around the circular chromosome until the two replication forks meet in the terminus region (Ter), located approximately opposite the origin. This eventually yields two copies of the bacterial chromosome, each containing one strand from the parental chromosome and one nascent strand Egan [60].
The origin contains a series of five 9-base pair sequence repeats known as DnaA (or R) boxes, to which DnaA-ATP and DnaA-ADP bind, as well as three additional sites (I boxes) that are specific for the ATP bound form Mott & Berger [61]. At the onset of a round of DNA replication, binding of ATP-bound DnaA molecules to the remaining sites (R3, I1, I2 and I3) leads to separation of the two template DNA strands at a nearby AT-rich region. Following strand separation, one ring-shaped hexamer of the replicative helicase DnaB (DnaB6) is loaded onto each of the DNA strands in the same orientation and each proceeds to unwind the parental DNA duplex, creating replication forks that move away from the origin in opposite directions. The replicase, DNA polymerase III holoenzyme (Pol III HE), associates with the forks and synthesizes both new DNA strands, leaving two completed duplex structures in its wake. Like most DNA polymerases, Pol III cannot begin DNA synthesis on a single-stranded DNA template; it can only extend preexisting DNA or RNA primers. It is the DnaG primase that first associates with DnaB at the replication fork and constructs short RNA primers, which are then extended by Pol III to build each new DNA strand Leonard and Grimwade [62].
Synthesis on the lagging strand occurs in four stages. Firstly, DnaG primase associates with the DnaB helicase (which translocates on the lagging strand), recognizes a trinucleotide recognition sequence, and produces an RNA primer (of up to 14 nucleotides). This happens about once every 1000 nt during lagging strand synthesis. A sliding clamp is loaded at the newly primed site by the clamp loader complex, onto which a Pol III core then associates. The Pol III core synthesizes new DNA until it reaches the preceding primer, producing a 1 kb Okazaki fragment. The Pol III core is then transferred onto a subsequent (upstream) β-loaded primer to begin synthesizing a new fragment. The RNA primers between fragments are removed by DNA polymerase I and replaced with DNA by the latter enzyme. Finally, the DNA fragments are joined by DNA ligase to create a continuous lagging DNA strand (Figure 13 & 14), Mott &Berger [61].
Of all the antibiotics in current clinical use, only two classes inhibit the process of DNA replication. The quinolone and aminocoumarin drugs inhibit the action of DNA gyrase, a type II topoisomerase that introduces negative supercoils in DNA ahead of replication forks to enable continued strand separation by the helicase during DNA replication. Given that the replication machinery includes so many other proteins with functions essential for bacterial viability, it is likely that at least some are useful drug targets. The replisome is very much an underexplored target for drug development, however, and few attempts to discover specific inhibitors have been described. One of the major challenges in discovering DNA replication inhibitors has been the need for biochemical assays that can be used for highthroughput screening. Important advances have been made in this area recently and are likely to yield new inhibitors soon, Black & Coleman [63].
1.1. DNA Gyrase Inhibitors
Gyrase (a type IIA DNA topoisomerase) plays an essential role in DNA replication, actively underwinding the double-stranded template DNA ahead of the replication fork to obviate effects of positive supercoiling induced by the progressing replisome. It acts by cutting both DNA strands, passing double-stranded DNA through the gap, and then re-ligating the original ends. It is a heterotetrametric enzyme comprised of two different subunits: GyrA, which binds DNA and carries out strand cleavage/ligation, and GyrB, which hydrolyses ATP to drive the supercoiling reaction Sissi & Palumbo [64].
Gyrase is the cellular target of several different classes of bactericidal agents, including the highly successful fluoroquinolone antibiotics, coumarins and cyclothialidines that are currently undergoing drug development, as well as a range of naturally occurring bacterial toxins Bradbury & Pucci [65]. Much of the current research on gyrase inhibitors focuses on modifying existing scaffolds to address issues with drug resistance, toxic side effects and poor cellular penetration, as well as to broaden the range of bacterial species that compounds are active against. Structure-aided design and virtual screening techniques have proven somewhat successful in delivering new classes of gyrase inhibitors, some of which show broad-spectrum antibacterial activity and reduced rates of resistance relative to existing drugs Ostrov [66].
Most recently, there has been exciting progress with two novel classes of gyrase inhibitors that show great promise for development into new antibiotics soon. The novel antibiotic simocyclinone D8 (Figure 15) was originally identified in extracts from the antibiotic-producing bacterium Streptomyces antibioticus Tü 6040 Schimana [67]. This compound has a chlorinated aminocoumarin group linked to an angucyclic polyketide moiety through a tetraene linker and a Dolivose sugar. The presence of the aminocoumarin group suggested DNA gyrase to be the cellular target and simocyclinone D8 was shown to potently inhibit the E. coli enzyme in vitro. Unlike the aminocoumarin antibiotics however, simocyclinone D8 was found not to inhibit the ATPase activity of the GyrB subunit, nor did it stimulate the formation of unproductive cleavage complexes within the GyrA subunit as seen with quinolone compounds, suggesting an entirely novel mode of inhibition Flatman [68].
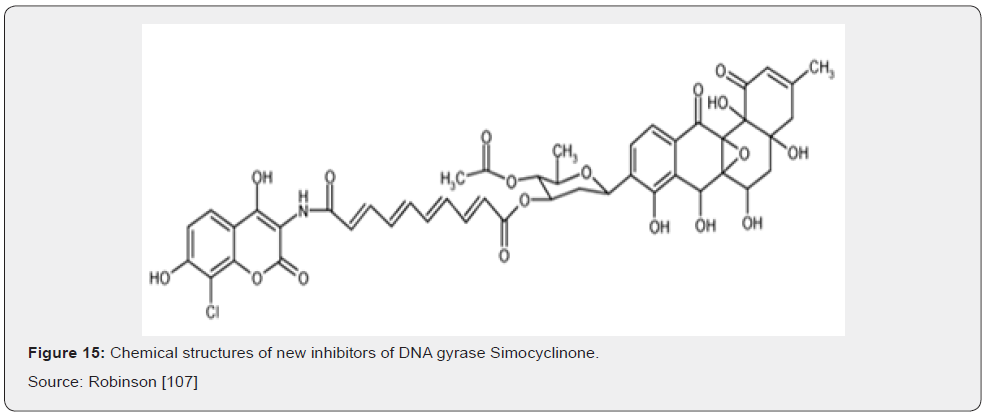
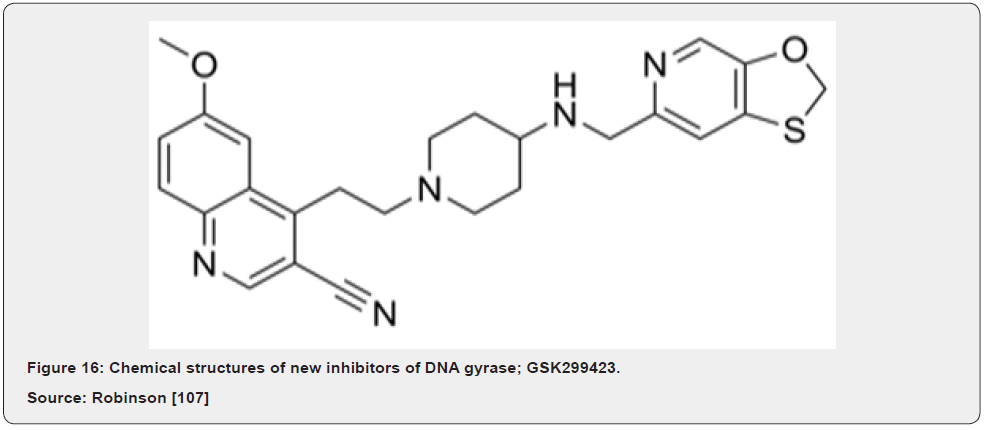
A very promising new antibiotic that acts on gyrase has been recently described by GlaxoSmithKline researchers; GSK299423 (Figure 16) is a member of the novel bacterial topoisomerase inhibitors (NBTI) family of compounds. GSK299423 strongly inhibits the activity of S. aureus and E. coli gyrase in vitro (IC50 = 14–100 nM), making it more than 2000 times more potent than the widely used drug ciprofloxacin (fluoroquinolone). GSK299423 shows potent antibacterial activity against a range of Gram-positive and Gram-negative pathogens (MIC = 0.016–8 μg/mL), including strains that are resistant to fluoroquinolones. GSK299423 appears to inhibit the catalytic cycle of gyrase by stabilizing a precleavage enzyme-DNA complex, an entirely novel mode of gyrase inhibition. Taken together, the potency of GSK299423, its broadspectrum activity and its lack of cross-resistance with existing fluoroquinolones suggest the exciting candidates for development into a much-needed new class of antibiotics with a novel mode of action Bax [69].
Inhibitors of DNA Polymerase Activity
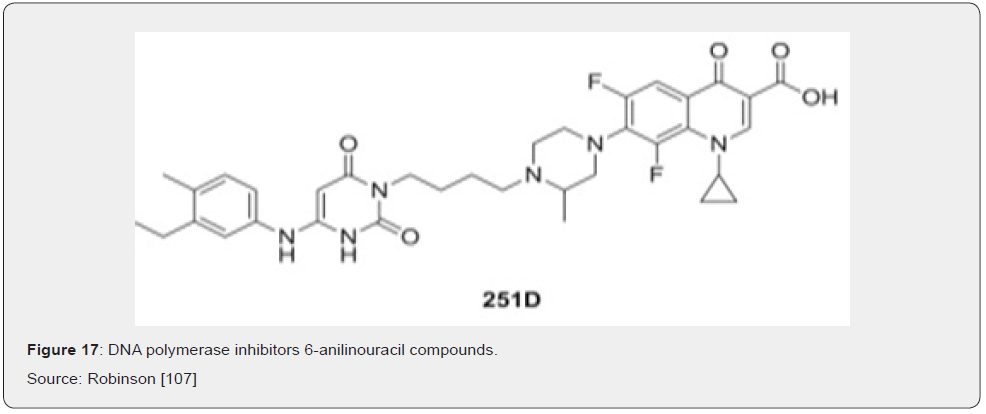
DNA polymerases are the targets of several important anti-viral and anti-cancer drugs, yet few inhibitors exist for the equivalent bacterial enzymes Berdis [70]. Two classes of compounds are known to effectively inhibit the PolC-type polymerases: 6-anilinouracils (6-AUs) and quinazolin-2-ylamino-quinazolin-4-ols (Bis Quinols). Members of a third class of compounds, the dichlorobenzylguanines, have been shown to inhibit the activities of DnaE1-, DnaE3- and PolC-type polymerases, although no analysis of their antibiotics properties has been published since their syntheses were described six years ago Wright [71]. The 6-anilinouracils are the oldest and most developed class of DNA polymerase inhibitors Brown [72]. These compounds are competitive inhibitors of dGTP binding to PolC, forming a threehydrogen- bond base pair with cytosine residues in the template DNA (Figure 17). Many variations have been made on the original 6-AU scaffold, producing several compounds that potently inhibit PolC activity and bacterial growth in vitro Butler [9].
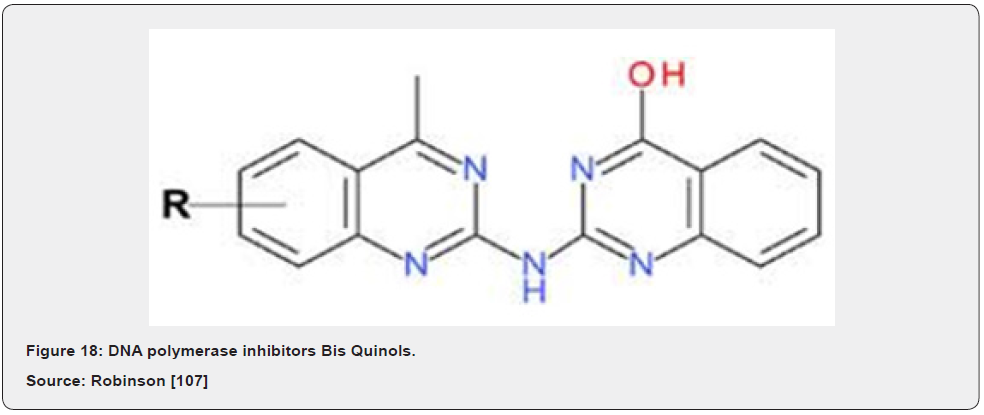
BisQuinols were discovered recently using high-throughput fluorescence-based inhibition assays. It was predicted that BisQuinols might base pair with cytosine residues in DNA templates and thus be competitive with dGTP substrates, as observed for the 6-AUs. Biochemical analysis revealed, however, that BisQuinols were uncompetitive with respect to nucleotide substrates, instead showing competitive behavior against template DNA. The mechanism of PolC inhibition by BisQuinols must therefore be different to that of 6-AU compounds and appears to be more analogous to that of non-nucleoside reverse transcriptase inhibitors used to treat HIV infections Guiles [73] (Figure 18). A promising new strategy for the development of PolC inhibitors is to covalently link 6-anilinouracil derivatives to fluoroquinolones to create hybrid PolC-DNA gyrase inhibitors.
These hybrid compounds maintain dual anti-PolC and anti-DNA gyrase activity in vivo and have greater or equal potency against whole bacterial cells than their isolated 6- AU or fluoroquinolone parent compounds. The use of hybrid inhibitors intuitively should provide greater protection against the development of drug resistance by target mutagenesis than traditional single-target inhibitors; this would require co-mutation of two loci in the bacterial population Zhi [74].
Inhibitors of Primosome Functions
Outside of DNA gyrase and the replicative polymerases, another replication subcomplex to be targeted for drug discovery has been the primosome, comprised of the DnaB/C helicase and DnaG primase. These proteins play critical roles in lagging strand synthesis and are essential for bacterial growth. These proteins are targets for some naturally occurring DNA replication inhibitors. The dietary flavonoid myricetin, for instance, has antimicrobial activity and has been shown to potently inhibit the replicative helicase, but not primase. The bacterial alarmone compound (p) ppGpp on the other hand, is now known to inhibit the activity of primase, thereby halting DNA replication as part of the stringent response (Macig et al., 2010). In another similar study conducted by Schering Plough researchers, a novel bicyclic macrolide compound, Sch 642305, was isolated from the fungus Penicillium verrucosum and found to inhibit E. coli primase invitro Chu [75].
Inhibitors of the Initiation of DNA Replication
A relatively new approach towards the discovery of DNA replication inhibitors is to target the initiation stage. In (almost) all bacteria, the first step in replicating the chromosome is the binding of multiple DnaA molecules at the origin, which ultimately leads to separation of the two template strands. Since this step signifies commitment to a complete round of replication, the amount and activity of DnaA is tightly regulated within the cell. Insufficient DnaA leads to under-initiation, while excess leads to over-initiation, both disrupt cell growth. Currently, no antibacterial agents target the initiation process Kaguni [76].
Fossum and colleagues have recently developed a robust cellbased assay to screen for inhibitors of DnaA activity Fossum [77]. The assay utilizes an E. coli strain containing a novel dnaA allele that produces an over-active DnaA variant and confers a coldsensitive phenotype. This strain grows well at the permissive temperature (42˚C) but grows poorly at 30˚C due to excessive initiation of DNA replication. Compounds that inhibit DnaA activity reduce the initiation rate and thus restore cell growth at 30˚C. This was demonstrated by expressing moderate levels of the DnaA N-terminal domain, a competitive inhibitor of full-length DnaA, within the cells. While a small-scale screen of microbial extracts failed to identify any inhibitors of DnaA activity, the simplicity of this assay would easily facilitate larger screens and could lead to the discovery of inhibitors in the future Kaguni [76].
A second cell-based assay has been developed recently with the aim of discovering inhibitors of replication initiation in Vibrio spp. Mandal and Mandal [78]. This group of bacteria includes the causative agent of cholera, common sources of food poisoning and pathogens of economically important marine animals. Unlike most bacteria, Vibrio spp. contains two chromosomes, only one of which is replicated in a DnaA dependent manner. Initiation of replication at the second chromosome relies on the activity of a second protein, RctB that bears no sequence similarity to DnaA. Yamaichi and colleagues established a cell-based screen for identifying inhibitors of V. cholerae RctB Mandal and Mandal [78]. The assay uses an E. coli strain that carries an RctB-dependent plasmid expressing both RctB and a kanamycin-resistance marker. If RctB is inhibited by a compound the cells cannot replicate the plasmid and thus do not grow on kanamycin-containing media. Using this strain, the group screened a library of 138,000 small molecules, identifying a potent inhibitor of RctB, vibrepin that inhibited the growth of all tested Vibrio spp. but had no effect on the growth of wild-type E. coli cells. If RctB inhibitors such as vibrepin can be developed into functional antibiotics, they should only kill the infectious Vibrio spp. whilst not affecting the natural gut flora of a patient, offering a significant advantage over current treatment options Mandal & Mandal [78].
Antibiotic Resistance in Ethiopia
The emergence of antibiotics resistance is a result of the use, overuse, and misuse of antibiotics both in human and animals’ health practices. In Ethiopia, there were indications on the misuse of antibiotics by health care providers, unskilled practitioners, and drug consumers. This coupled with rapid spread of resistant bacteria and inadequate surveillance contributed to the problem. Studies on antibacterial resistance and on bacterial infections have shown that emerging antibacterial resistance threatens the management of bacterial infections. However, prevention and containment has received far too little attention. The consequences of this phenomenon include increased mortality, morbidity, costs of treatment, loss of production in animals and death DACAE & MSH [79]. In Ethiopia the control of drugs from the government authorities and information on the rational use of drugs pertaining to veterinary drug is very limited. In addition, there is lack of awareness and preparedness among the controlling authorities and producers in dealing with the risk of indiscriminate use of antibiotics to the livestock and to the consumers. No formal control mechanisms exist to protect the consumers against the consumption of meat and milk products containing harmful drug residues in the country Hunde [80].
The most health workers do not adhere to the rational antimicrobial prescription guidelines. The diagnosis of a disease is generally presumptive; drug sensitivity tests are not carried out and the selection of drugs is primarily based on their availability rather than their efficacy. In addition, a few antibiotics were commonly prescribed and factors that could help reduce the rates of emergence of resistant pathogens were not considered by most respondents. Moreover, verbal prescription is the major form of prescription and the prescription papers used in some clinics do not contain all the relevant information. In a study on the antimicrobial resistance features of salmonella isolates of dairy cattle in Addis Ababa, almost all isolates (20/21) were multi drug resistant Addis [81]. The irrational use of drugs is a major problem in present day clinical practices as it could result in toxicities and treatment failures in patients and in the emergence of drug resistant pathogens. Whilst drug resistant bacteria were traditionally acquired in hospitals due to high antibiotic use and disease transmission rate, community acquired drug resistant bacteria are becoming increasingly common. Resistance may escalate to the point at which the efficacy of drugs will no more be predictable and infections once treatable could become untreatable FMOH & WHO [82].
In addition, the most common problems for the increasing of antibiotic resistance, in Ethiopian situation, are weak storage capacity of pharmaceuticals, long stock-outs of essential drugs in public health facilities, widespread over the counter (OTC) availability of antibiotics, poor dispensing, drug quality concerns, especially in rural areas, unregistered drugs and drug sellers in rural areas, low patient knowledge about drugs dispensed to them and weak monitoring and lack of antibiotic policy and evaluations FMOH and WHO [82] (Table 2).
Prevention of Antibiotic Resistances
Responsible Use
Guidelines exist for responsible (proper, appropriate, prudent, or judicious) use of antibiotics in veterinary and human medicine and are similar in the medical and agricultural sectors. Veterinary and animal producer organizations in many countries have developed and implemented use of guidelines. These addresses are used in various species, including poultry, swine, dairy and beef cattle, and sheep. International organizations, such as the OIE and WHO, also have developed principles or codes of practice to contain antibiotic resistance. The WHO published global principles for the containment of antibiotics resistance in animals intended for food. The OIE issued five documents concerning antibiotic resistance, including guidelines for the responsible and prudent use of antibiotics agents in Veterinary Medicine WHO [12].
Alternative Practice
Herd, flock, and other health management programs overseen by veterinary or other professionals attempt to minimize infectious disease outbreaks by using non-antibiotic interventions early in the life of the animals such as good hygiene practice. The rationale is to promote healthy animals that do not become ill and are, thus, unlikely to be treated with an antibiotic agent. Several current approaches are available. These non-antibiotic approaches have led to a need to establish performance standards for regulatory and commercial purposes Rose [32].
Preventing Infectious Diseases in Animals
Focus should be given to the continuous implementation of appropriate measures for disease prevention, to decrease the need for antibiotics. To minimize infection in food animal production and decrease the amount of antibiotics used, efforts should aim to improve animal health, thereby eliminating or reducing the need for antibiotics for treatment or prophylaxis. This can be achieved by improving hygiene, biosecurity and health management on farms and preventing disease using vaccines and other measures such as probiotics (beneficial bacteria found in various foods), prebiotics (non-digestible foods that help probiotic bacteria grow and flourish) or competitive exclusion products (intestinal bacterial flora that limit the colonization of some bacterial pathogens) Kung [83].
The use of Competitive exclusion in which direct-fed microbial products containing live microorganisms (known as probiotics) or products containing enzymes as the active ingredient are currently marketed in many countries. Probiotics, which contain one or more types of microorganisms and are administered orally, are currently approved for use in food animals in Europe and other countries, but as for the use of antibiotics for growth promotion, their mode of action is not fully understood. Probiotic bacteria could affect normal gut microflora by competitive exclusion of pathogenic bacteria, production of antibacterial products or enzymes that act on gut bacteria, or production of other metabolites that affect gut commensals Kung [83].
Conclusion and Recommendations
Antibiotics are extensively used both in human and animal health practices in developed and developing countries of the world mainly for treatment and control of various diseases and as feed additives. However, the residue, use, misuse, and overuse of these medicines contributed favorable conditions for the emergence, occurrence, and development of antibiotic resistant bacteria. Similarly, the other factor which contributes for includes Sub-therapeutic doses, non-laboratory oriented antibiotic therapy, use of ineffective drugs and poor storage of drugs. These all can result in infections with much more difficult to treat. Even though there are guidelines in both human and veterinary medicine for responsible use of antibiotics, vaccination, competitive exclusions and others for rational use and the control of antibiotic resistance, there are still some indications on the misuse of antibiotics by health care providers, unskilled practitioners, and drug consumers. These all coupled with rapid spread of resistant bacteria, consequently, may lead to increased mortality, morbidity, costs of treatment, and loss of production in animals. Even though the effect of antibiotic resistance is magnificent, there is still inadequate surveillance and far little attention on rational use of drugs to minimize antibiotic resistance. One of the most alarming consequences of this flawed system is the failure to develop new antibiotics to combat multidrug-resistant bacteria and the problem of antibiotics resistance calls attention to the pressing need to reform our outdated model of pharmaceutical innovation. So based on the above conclusion the following recommendations are forwarded:
a) Awareness creation should be conducted on rational use of drugs for the community and other stake holders.
b) Antibiotic treatment should be provided after isolation, identification and conduction of drug sensitivity test and use of antibiotics as growth promoters should be prohibited.
c) Narrow spectrum antibiotics should be the first choice when antibiotic therapy is justified.
d) Research needs to be developed on the best ways to mitigate the development of antibiotic resistance. Research needs on development of novel antibiotic drug against bacteria that develop antibiotic resistance on DNA replication with this in mind [84-111].
References
- Flowers C, Seidenfeld J, Bow E, Karten C, Gleason C, et al. (2015) Antimicrobial Prophylaxis and Outpatient Management of Fever and Neutropenia in Adults Treated for Malignancy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 31(6): 794-810.
- Nigel C Rollins MD (2016) Why invest, and what it will take to improve breastfeeding practices. The Lancet Journal 387(10017): 491-504.
- Joshi S (2002) HPLC separation of antibiotics present in formulated and unformulated samples. J Pharm Biomed Anal 28(5): 795-809.
- Bow E (2013) Infection in Neutropenaic Patients with Cancer. Crit Care Clin 29: 411-441.
- Zeina K, Pamela A, Fawwak S (2013) Quantification of Antibiotic Residues and Determination of Antimicrobial Resistance Profiles of Microorganisms Isolated from Bovine Milk in Lebanon. J Food Nutr Sci 4(7A): 1-9.
- Alla W, Mohamed E, Abdelgadir E (2011) Detection of antibiotics residues in beef in ghnawa slauterhouse, khartoum state, sudan. Vet Med Anim Prod 2: 71-88.
- Dubois M, Fluchard D, Sio E, Delahaut P (2001) Identification and quantification of five macrolide antibiotics in several tissues, eggs, and milk by liquid chromatography – electrospray tandem mass spectrometry. J Chromatogr 753(2): 189-202.
- Davies J, Davies D (2010) Origins and Evolution of Antibiotic Resistance. Microbiol Mol Biol Rev 74(3): 417-433.
- Butler C, Hillier S, Roberts Z, Dunstan F, Howard A, et al. (2006) Antibiotic-resistant infections in primary care are symptomatic for longer and increased workload: outcomes for patients with E. coli UTIs. Br. J Gen Pract 56: 686-692.
- Sarmah A, Meyer M, Boxall A (2006) A global perspective on the use, sales, exposure pathways, occurrence, fate and effects of veterinary antibiotics (VAs ) in the environment. J Chemosphere 65(5): 725-759.
- CDC (2013) National Antimicrobial Resistance Monitoring System Human Isolates Final Report. Hum Isol Final Rep.
- WHO (2014) Antimicrobial resistance: global report on surveillance. Glob Rep Surveill.
- McDermott F, Walker R (2016) Antimicrobials: Modes of Action and Mechanisms of Resistance. Int J Toxicol 22(2): 135-143.
- Xu H, Qin S, Liu H (2014) New Synthetic Antibiotics for the Treatment of Enterococcus and Campylobacter Curr Top Med Chem 14(1): 21-39.
- Yousef F, Mansour O, Jehad H (2016) Sulfonamides: Historical Discovery Development (Structure-Activity Relationship Notes). In-vitro In-vivo In-silico J 1(1): 1-15.
- Ali J, Rafiq Q, Ratcliffe E (2018) Antimicrobial resistance mechanisms and potential synthetic treatments. J Futur Sci OA 4(4): 290.
- Reynolds P (1989) Structure, Biochemistry and Mechanism of Action of Glycopeptide Antibiotics CO- CO- CO-. Eur J Clin Microbiol Infect Dis 8(11): 943-950.
- Džidic S, Šuškovic J (2008) Antibiotic Resistance Mechanisms in Bacteria: Biochemical and Genetic Aspects. J Food Technol Biotechnol 46(1): 11-21.
- Johnston N, Mukhtar T, Wright G (2002) Streptogramin Antibiotics: Mode of Action and Resistance. Curr Drug Targets 3(4): 335-344.
- Vannuffel P, Cocito C (1996) Mechanism of Action of Streptogramins and Macrolides. J Drugs 51: 20-30.
- Yoneyama H, Katsumata R (2006) Antibiotic Resistance in Bacteria and Its Future for Novel Antibiotic Development. Biosci Biotechnol Biochem 70(5): 1060-1075.
- Smith D, Dushoff J, Morris J (2005) Agricultural Antibiotics and Human Health Does antibiotic use in agriculture have a greater impact than hospital use? PLoS Med 2(8): 232.
- Amenu D (2014) Antimicrobial Resistance for Enteric Pathogens Isolated from Acute Gastroenteritis Patients. World J Nat Appl Sci 1: 1-14.
- Bennett P (2008) Plasmid encoded antibiotic resistance: acquisition and transfer of antibiotic resistance genes in bacteria. Br J Pharmacol 153: 347-357.
- FWW (2015) Antibiotic Resistance 101: How Antibiotic Misuse on Factory Farms Can Make You Sick About Food & Water Watch. Food Water Watch p. 1-17.
- European Commission (2010) Commission staff working document on the implementation of national residue monitoring plans in the Member States in 2010 (Council Directive 96/23/EC).
- FDA (2011) Chapter 11: aquaculture drugs understand the potential hazard.
- Marshall B, Levy S (2011) Food Animals and Antimicrobials: Impacts on Human Health Food Animals and Antimicrobials: Impacts on Human Health. J Clin Microbiol Rev 24: 718-733.
- Dugassa J (2018) Review of antibiotic resistance and its mechanism of development. J Heal Heal Med Nurs 1(3): 1-17.
- Levy S (2002) Factors impacting on the problem of antibiotic resistance. J Antimicrob Chemother 49(1): 25-30.
- Marti E, Variatza E, Balcazar J (2014) The role of aquatic ecosystems as reservoirs of antibiotic resistance. Trends Microbiol 22: 36-41.
- Rose J, Gast R, Bogomolni A, Ellis J, Lentell B, et al. (2016) Antibiotics Versus Appendicectomy for the Treatment of Uncomplicated Acute Appendicitis: An Updated Meta-Analysis of Randomized Controlled Trials. World J Surg 40: 2305-2318.
- Rafailidis P, Pitsounis A (2009) Meta-analyses on the Optimization of the Duration of Antimicrobial Treatment for Various Infections. J Infect Dis Clin North Am 23: 269-276.
- Moran G, Amii R, Abrahamian F, Talan D (2005) Methicillin-resistant Staphylococcus aureus in community-acquired skin infections. J Emerg Infect Dis 11(6): 928-930.
- Araya P, Hug J, Joy G, Oschmann F, Rubinstein S (2016) The impact of water and sanitation on diarrhoeal disease burden and over-consumption of antibiotics. Master Public Adm London Sch Econ Polit Sci
- Silbergeld E, Graham J, Price L (2008) Industrial Food Animal Production, Antimicrobial Resistance, and Human Health. Annu Rev Public Heal 29: 151-69.
- Kieninger A, Lipsett P (2009) Hospital-Acquire d Pneumonia: Pathophysiology, Diagnosis, and Treatment. J Surg Clin NA 89: 439-461.
- D’Costa1 V, King C, Kalan L, Morar M, Sung W, et al. (2011) Antibiotic resistance is ancient. J Nature 477: 457-461.
- Wright G, Brown N, Xu W, Long Z, Zhi C, et al. (2005) Active site directed inhibitors of replication-specific bacterial DNA polymerases. Bioorg Med Chem Lett 15(3): 729-732.
- Courvalin P (2005) Antimicrobial Drug Resistance: Prediction Is Very Difficult, especially about the Future. J Emerg Infect Dis 11(10): 1503-1506.
- Clewell D (2014) Antibiotic Resistance Plasmids in Bacteria. Elsevier 10: 1-6.
- Hegstad K, Mikalsen T, Coque T, Werner G, Sundsfjord A (2010) Mobile genetic elements and their contribution to the emergence of antimicrobial resistant Enterococcus faecalis and Enterococcus faecium. Clin Microbiol Infect 16(6): 541-554.
- Langton K, Henderson P, Herbert R, Langton K (2005) Antibiotic resistance: multidrug efflux proteins, a common transport mechanism? J Nat Prod Rep 22: 439-451.
- Sageman A (2015) Antibiotic Resistance Mechanisms, Problems, and Solutions. Med Heal Sci Commons pp.416.
- Holcomb H, Urbin K, Cho M, Choi K, Darling N, et al. (2008) Methicillin-Resistant Staphylococcus aureus as a Threat to Public Health: A Cellular Approach. J Heal Sci Georg Univ 5(2): 1-8.
- Hancock R, Brinkman F (2002) Function of pseudomonas porins in uptake and efflux. Annu Rev Microbiol 56: 17-38.
- Nikaido H (2003) Molecular Basis of Bacterial Outer Membrane Permeability Revisited Molecular Basis of Bacterial Outer Membrane Permeability Revisited. J Microbiol Mol Biol Rev 67(4): 593-656.
- Wilson D (2014) Ribosome-targeting antibiotics and mechanisms of bacterial resistance. Nat Publ Gr 12(1): 35-48.
- Ramirez M, Tolmasky M (2010) Aminoglycoside modifying enzymes. Drug Resist Updat 13: 151-171.
- Schwarz S, Kehrenberg C, Cloeckaert A (2004) Molecular basis of bacterial resistance to chloramphenicol and florfenicol. Fems Microbiol Rev 28(5): 519-542.
- Jose M, Cesar A (2016) Mechanisms of Antibiotic Resistance. J Microbiol Spectr 4: 1-37.
- Tenover F (2006) Mechanisms of Antimicrobial Resistance in Bacteria. Am J Med 119: 3-10.
- Grundmann H, Aires-de-sousa M, Boyce J, Tiemersma E (2006) Emergence and resurgence of meticillin-resistant Staphylococcus aureus as a public-health threat. J Lancet 268: 874-885.
- Kim Y, Cha C, Cerniglia C (2002) Purification and characterization of an erythromycin esterase from an erythromycin-resistant Pseudomonas sp. J FEMS Microbiol 210: 239-244.
- Willey J, Sherwood L, Woolverton C (2017) Prescott’s Microbiology. 10th (Ed).
- Bbosa G, Mwebaza N, Odda J, Kyegombe D, Ntale M (2014) Antibiotics/antibacterial drug use, their marketing and promotion during the post-antibiotic golden age and their role in emergence of bacterial resistance. J Heal 6(5): 410-425.
- Bockstael K, Aerschot A (2014) Antimicrobial resistance in bacteria. J Cent Eur J Med 4(2): 141-155.
- Jim O (2014) Review on Antimicrobial Resistance. Antimicrobial Resistance: Tackling a Crisis for the Health and Wealth of Nations.
- Ahmed A, Barış E, Delfin G, Hans L, Israel O, et al. (2017) Assessing the Global Economic and Poverty Effects of Antimicrobial Resistance. Policy Res Work Pap Rep 10: 8133.
- Egan E, Fogel M, Waldor M (2005) Micro Review Divided genomes: negotiating the cell cycle in prokaryotes with multiple chromosomes. Mol Microbiol 56: 1129-1138.
- Mott M, Berger J (2007) DNA replication initiation: mechanisms and regulation in bacteria. J Nat Rev Microbiol 5: 343-354.
- Leonard A, Grimwade J (2006) Building a bacterial orisome: emergence of new regulatory features for replication origin unwinding. J Mol Microbiol 55: 978-985.
- Black M, Coleman K (2016) New inhibitors of bacterial topoisomerase GyrA / ParC subunits. J Curr Opin Investig drugs 10(8): 804-810.
- Sissi C, Palumbo M (2010) In front of and behind the replication fork: bacterial type IIA topoisomerases. Cell Mol Life Sci 67: 2001-2024.
- Bradbury B, Pucci M (2008) Recent advances in bacterial topoisomerase inhibitors. Curr Opin Pharmacol 8: 574-581.
- Ostrov D, Prada J, Patrick E, Finton K, Le N, et al. (2007) Discovery of Novel DNA Gyrase Inhibitors by High-Throughput Virtual Screening. J Antimicrob Agents Chemother 51: 3688-3698.
- Schimana J, Fiedler H, Institut M, Tubingen U, Hannover D, et al. (2000) Simocyclinones, Novel Cytostatic Angucyclinone Antibiotics Produced by StrSimocyclinones, oticus Tii 6040 I. Taxonomy, Fermentation, Isolation and Biological Activities. T J Antibiot (Tokyo) 53: 779-87.
- Flatman R, Howells A, Heide L, Fiedler H, Maxwell A et al. (2005) Simocyclinone D8, an Inhibitor of DNA Gyrase with a Novel Mode of Action. Antimicrob. Agents Chemother 49: 1093-1100.
- Bax B, Chan P, Eggleston D, Fosberry A, Gentry D, et al. (2010) Type IIA topoisomerase inhibition by a new class of antibacterial agents. Nature 466: 935-940.
- Berdis A (2008) Current Topics DNA Polymerases as Therapeutic Targets. J Biochem 12: 8253-8260.
- Wright G (2010) Antibiotic resistance in the environment: a link to the clinic? Curr Opin Microbiol 13: 589-594
- Brown N (1970) 6- (p-Hydroxyphenylazo) -uracil: A Selective Inhibitor of Host DNA Replication in Phage-Infected Bacillus subtilis. Proc Natl Acad Sci 67: 1454-1461.
- Guiles J, Sun X, Critchley I, Ochsner U, Tregay M, et al. (2009) Bioorganic & Medicinal Chemistry Letters Quinazolin-2-ylamino-quinazolin-4-ols as novel non-nucleoside inhibitors of bacterial DNA polymerase III AU. Bioorg Med Chem Lett 19: 800-802.
- Zhi C, Long Z, Manikowski A, Brown N, Tarantino P, et al. (2005) Synthesis and Antibacterial Activity of 3-Substituted-6- (3-ethyl-4-methylanilino) uracils. J Med Chem 48: 7063-7074.
- Chu M, Mierzwa R, Xu L, He L, Terracciano J, et al. (201) Primase Inhibitor Produced by Penicillium verrucosum. J Nat Prod 66: 143-145.
- Kaguni J (2006) DnaA: Controlling the Initiation of Bacterial DNA Replication and More. J Annu Rev Microbiol 60: 351-75.
- Fossum S, Pascale G, Weigel C, Messer W, Donadio S, et al. (2008) Research letter a robust screen for novel antibiotics: specific knockout of the initiator of bacterial DNA replication. FEMS Microbiol Lett 281: 210-214.
- Mandal S, Mandal S (2009) Rational drug design. Eur J Pharmacol 625: 90-100.
- DACAE and MSH (2009) Antimcirobials use, resistance and containment baseline survey syntheses of findings. A report published by Drug Administration and Control Authority of Ethiopia. Addis Ababa, Ethiopia.
- Hunde A, Zewde B, Zewde B (2012) Tetracycline Residue Levels in Slaughtered Beef Cattle from Three Slaughterhouses in Central Ethiopia. J Global Vet 8: 546-554.
- Addis Z, Kebede N, Worku Z, Gezahegn H, Yirsaw A, et al. (2011) Prevalence and antimicrobial resistance of Salmonella isolated from lactating cows and in contact humans in dairy farms of Addis Ababa: a cross sectional study. BMC Infect Dis 11: 222.
- FMOH and WHO (2003) Ethiopia and WHO, Assessment of pharmaceutical sectors in Ethiopia.
- Kung L (2006) Direct fed microbial, enzyme and forage additive compendium. Anim Biotechnol 13: 97-112.
- Assefa A, Gelaw B, Shiferaw Y, Tigabu Z (2013) Nasopharyngeal carriage and antimicrobial susceptibility pattern of Streptococcus pneumoniae among pediatric outpatients at Gondar University hospital, northwest Ethiopia. Pediatr Neonatol 54: 315-321.
- Abera B, Kibret M (2014) Azithromycin, fluoroquinolone, and chloramphenicol resistance of non-chlamydia conjunctival bacteria in rural community of Ethiopia. Indian J Ophthalmol 62: 236.
- Tesfaye T, Beyene G, Gelaw Y, Bekele S, Saravanan M (2013) Bacterial profile and antimicrobial susceptibility pattern of external ocular infections in Jimma University specialized hospital, Southwest Ethiopia. Am J Infect Dis Microbiol 1: 13-20.
- Reta A, Bitew Kifilie A, Mengist A (2019) Bacterial Infections and Their Antibiotic Resistance Pattern in Ethiopia: A Systematic Review. Adv Prev Med 10: 1-5.
- Ramos J, Pérez-Tanoira R, García-García C, Prieto-Pérez L, Bellón MC, et al. (2014) Leprosy ulcers in a rural hospital of Ethiopia: pattern of aerobic bacterial isolates and drug sensitivities. Ann Clin Microbiol Antimicrob 13: 47.
- Dagnew M, Yismaw G, Gizachew M, Gadisa A, Abebe T, et al. (2013) Bacterial profile and antimicrobial susceptibility pattern in septicemia suspected patients attending Gondar University Hospital, Northwest Ethiopia. BMC Res 6: 283.
- Muluye D, Wondimeneh Y, Moges F, Nega T, Ferede G (2014) Types and drug susceptibility patterns of bacterial isolates from eye discharge samples at Gondar University Hospital, Northwest Ethiopia. BMC Res 7: 292.
- Mengesha R, Kasa B, Saravanan M, Berhe D, Wasihun A (2014) Aerobic bacteria in post-surgical wound infections and pattern of their antimicrobial susceptibility in Ayder Teaching and Referral Hospital, Mekelle, Ethiopia. BMC Res 7: 575.
- Teweldemedhin M, Saravanan M, Gebreyesus A, Gebreegziabiher D (2017) Ocular bacterial infections at Quiha Ophthalmic Hospital, Northern Ethiopia: an evaluation according to the risk factors and the antimicrobial susceptibility of bacterial isolates. BMC Infect Dis 17: 207.
- Getahun E, Gelaw B, Assefa A, Assefa Y, Amsalu A (2017) Bacterial pathogens associated with external ocular infections alongside eminent proportion of multidrug resistant isolates at the University of Gondar Hospital, northwest Ethiopia. BMC Ophthalmol 17: 151.
- Raja Duraipandi K, Mani K, Panneerselvam K, Mani M, Bhaskar M, et al. (2006) Prevalence and antimicrobial susceptibility pattern of methicillin resistant Staphylococcus aureus: A multicentre study. Indian J Med Microbiol 24: 34-38.
- Bitew A, Molalign T, Chanie M (2017) Species distribution and antibiotic susceptibility profile of bacterial uropathogens among patients complaining urinary tract infections. BMC Infect Dis 17: 654.
- Wasihun A, Wlekidan L, Gebremariam S, Dejene T, Welderufael A, et al. (2015) Bacteriological profile and antimicrobial susceptibility patterns of blood culture isolates among febrile patients in Mekelle Hospital, Northern Ethiopia. Spring J 4: 314.
- Abamecha A, Wondafrash B, Abdissa A (2015) Antimicrobial resistance profile of Enterococcus species isolated from intestinal tracts of hospitalized patients in Jimma, Ethiopia. BMC Res 8: 213.
- Seid J, Asrat D (2005) Occurrence of extended spectrum β-lactamase enzymes in clinical isolates of Klebsiella species from Harar region, eastern Ethiopia. Acta Trop 95: 143-148.
- Derbie A, Hailu D, Mekonnen D, Abera B, Yitayew G (2017) Antibiogram profile of uropathogens isolated at bahir dar regional health research laboratory centre, northwest ethiopia. Pan Afr Med J 26: 1-6.
- Wondimeneh Y, Muluye D, Alemu, Abebe, Atinafu A, et al. (2014) Urinary tract infection among obstetric fistula patients at Gondar University Hospital, Northwest Ethiopia. BMC Womens Health 14: 12.
- Mama M, Abdissa A, Sewunet T (2014) Antimicrobial susceptibility pattern of bacterial isolates from wound infection and their sensitivity to alternative topical agents at Jimma University Specialized Hospital, South-West Ethiopia. Ann Clin Microb Antimicr 13: 14.
- Eguale T, Gebreyes WA, Asrat D, Alemayehu H, Gunn JS, et al. (2015) Non-typhoidal Salmonella serotypes, antimicrobial resistance, and co-infection with parasites among patients with diarrhea and other gastrointestinal complaints in Addis Ababa, Ethiopia. BMC Infect Dis 15: 497.
- Etebu E, Arikekpar I (2016) Antibiotics: Classification and mechanisms of action with emphasis on molecular perspectives. Int J Appl Microbiol Biotechnol Res 4: 90-101.
- Megha S (2018) The hunt for new antibiotics grows harder as resistance builds. University of Chinese Academy of Sciences. Ther fsh sci p. 96.
- Bambeke V, Balzi E (2000) Antibiotic Efflux Pumps Franc. J Biochem Pharmacol 60: 457-470.
- Williams L, Detter C, Barry K, Lapidus A, Summers A (2006) Facile Recovery of Individual High-Molecular-Weight, Low-Copy-Number Natural Plasmids for Genomic Sequencing. Appl Env Microbiol 72: 4899-4906.
- Robinson A, Causer R, Dixon N (2012) Architecture and Conservation of the Bacterial DNA Replication Machinery, an Underexploited Drug Target. J Curr Drug Targets. 13: 352-372.
- H G, M A S, J B, Tiemersma E (2002) Non-typhoidal salmonella bacteremia among HIV- infected Malawian adults: high mortality and frequent recrudescence. J A I D S 16(12): 1633-1641.
- Morley P, Apley M, Besser T, Burney D, Fedorka P (2005) Antimicrobial drug use in veterinary medicine. American College of Veterinary Internal Medicine. J Vet Intern Med 19: 617-29.
- Nelson N, Joshi M, Kirika R (2009) Antimicrobial resistance: the need for action in the east, central and southern Africa region. Strength Pharm Syst Cent Pharm Manag Manag Sci Heal.
- O’Neill J (2014) Review of Antimicrobial Resistance. Antimicrobial Resistance: Tackling a Crisis for the Health and Wealth of Nations.






























