Nematicidal Activity of Goat Milk in Caenorhabditis elegans
Correia D S¹*, Lopes I S¹, Ferreira V M², Polveiro R C¹, Moreira M A S¹ and Araujo J V¹
1Department of Veterinary, Federal University of Viçosa, Viçosa, Brazil
2Veterinary Institute, Federal University of Rio de Janeiro, Seropédica, Brazil
Submission:March 01, 2023; Published:April 03, 2023
*Corresponding author: Douglas C Souza, Department of Veterinary, Federal University of Viçosa, Viçosa, Minas Gerais, Brazil
How to cite this article: Correia D S, Lopes I S, Ferreira V M, Polveiro R C, Moreira M A S and Araujo J V. Nematicidal Activity of Goat Milk in Caenorhabditis elegans. Dairy and Vet Sci J. 2023; 15(3): 555915.DOI: 10.19080/JDVS.2023.15.555915
Abstract
The high nematicidal activity found in milk from goats and other ruminants was recently elucidated with promising results in the control of enteroparasites in infected animals. However, it is still unclear which active molecules are involved and how this activity is distributed in samples from different animals. For an efficient use of these materials as nematicidal agents, it is necessary to verify this activity. The aim of this work was to evaluate Caenorhabditis elegans (C. elegans), a nematode model organism, as a bioindicator of this activity in the milk of different ruminants and how this potential is verified in a considerable sample of animals from the same species and different breeds and ages. Therefore, 200 goat milk samples were used in nematode challenge tests with readings at 24, 48, 72 and 96 hours of exposure. The average mortality of nematodes when exposed to goat milk was 74% in 24 hours, with a progressive average mortality over time reaching 98% in 96 hours. Alpine goats produced milk with lower levels of nematicity compared to other breeds after the 24-hour exposure (p=0.0919). Furthermore, milk from Saanen goats generated a higher average nematode’s mortality compared to other breeds in 48 hours (p=0.0078). C. elegans proved to be a good primary organism for analyzing the nematicidal activity of milk and to select samples with the best anthelmintic potential against parasitic nematodes.
Keywords:Ruminant Milk; Animal Health; Nematodes; Biological Control
Abbreviations:TBC: Total bacterial count; SCC: Somatic cell count; NGM: nematode growth medium
Introduction
Milk and its derivatives are nutritious sources of proteins, fats, micronutrients, prebiotics, and probiotics. The milk of certain ruminants has been highlighted as powerful agents with different antipathogenic properties. Hence, according to their functional properties, the so-called bioactive dairy products have already demonstrated activities such as anthelmintic, antimicrobial, antifungal, antithrombotic, antihypertensive, immunomodulatory, antioxidant and anti-inflammatory, according to recent research [1-7]. Several ruminants are used in extensive milk production, such as cattle, goats, and buffaloes. Milk and its derivatives promote positive results for health in general, so these various beneficial properties show a broad therapeutic potential to be explored. Another important point is that the use of these inputs has other indirect benefits, like the already established milk production industry that meets high standards of food safety, ensuring a safe product [8]. The goat’s milk market in Brazil is booming, showing significant growth in recent years, due to consumer demand in large urban centers, in addition to government actions in the Northeast Region, with the objective to improve families’ life’s quality through actions to combat hunger and child malnutrition. The Brazilian production of goat milk is around 26 million liters per year [9]. Diseases caused by parasitic helminths such as nematodes directly affect a quarter of the world’s population and affect all humans through infection in crops and in domestic and production animals. World billionaire losses are estimated because of intestinal parasites caused by nematodes in cattle, goats, and other production animals [10,11]. Drug-resistance and the limited number of anthelmintics available have led to the search for new alternatives [12]. The biological control of diseases using natural compounds is important because it has the potential to be an accessible, cheap, and effective alternative which can be used as a substitute to the classic agents or as an enhancer of it, reducing its use [13].
The free-living nematode C. elegans offers a convenient alternative model system to search for new compounds that specifically kill nematodes and by biological similarities may also be toxic to other helminths. C. elegans is about 1 mm long as an adult, it can be grown in a high-yield format for several generations, allowing the identification of molecules that disrupt the worm at any point in its life cycle and in multigeneration assays [14]. Therefore, among all its features, an undeniable contribution of C. elegans as a model organism is to constantly promote scientific innovations that have the potential to add new aspects to anthelmintic and other drugs research and expand our repertoire of techniques [15]. In the present study, the nematode model C. elegans was used as an alternative system to investigate the nematicity activity of goat milk. The objective was to evaluate the milk of different animals, which were grouped by age and breed. We also analyzed correlations with somatic cell count (SCC) and total bacterial count (TBC) levels markers commonly used to assess milk quality and aid in the diagnosis of mastitis.
Material and Methods
Milk samples
The milk from goats aged between 1 and a half and 8 years were analyzed. Thus, of the analyzed samples, 65 are from Saanen goats, 77 from Alpine and 58 crossbred animals, in total 200 samples were collected. These goats belong to the goat sector of the Department of Zootechnics of the Federal University of Viçosa. This sector is in Viçosa, Minas Gerais, 20° 46′22.8 ″ S 42° 51′10.8 ″ W. The animals are kept in intensive free-raising regime, with a high-level mechanical milking system and automatic cleaning of the milk tubes. Before milking, the teats were cleaned (pre-dip) with chlorine solution (750 ppm (Parts Per Million), dried with disposable towels and the first three jets of milk discarded in a “strip cup”. Milk sampling was performed by manual milking in sterile tubes. The samples were kept at 4°C until the time of analysis. The SCC and TBC analysis of goat’s milk were made by the Milk Quality Laboratory of EMBRAPA - CNPGL / Juiz de Fora - MG. The vials used to collect samples for the determination of SCC have their own identification with the preservative Bronopol® (2-bromo- 2-nitropropane-1,3-diol) inside. The bottles intended for TBC analysis used an azidiol preservative tablet (sodium azide and chloramphenicol) inside. Simultaneously, 10 samples of UHT (Ultra- High-Temperature Processing) cow’s milk purchased at the local market were used as a negative control due to their molecular similarity as a milk without high nematicide activity as previously tested.
Nematode culture and synchronization
C. elegans Bristol N2 and Escherichia coli (E. coli) OP50 from CGC (Caenorhabditis Genetics Center), were kindly donated by Prof a. Riva de Paula (UFRN). The worms were kept in a B.O.D incubator at 20°C in nematode growth medium (NGM) plates (1.7% Bacto agar, 0.5% Bacto peptone, 50 mM NaCl, 25 mM potassium phosphate buffer pH 6.0, 1 mM CaCl2, 1 mM MgSO4 and 5 μg/mL cholesterol, H2O to 1 liter) sown with LB broth incubated with E. coli OP50 strain as a food source and were manipulated using established techniques [16]. Briefly, to synchronize C. elegans with the same age and stage, pregnant adult worms were treated with sterile water containing 0.5 M NaOH and 4% bleach solution (NaOH) prepared at the time of use. The eggs were released and isolated by shaking, then centrifuged (1,300 × g for 1 min) and then washed 3 times in M9 buffer (3 g KH2PO4, 6 g Na2HPO4, 5 g NaCl, 1 ml 1 M MgSO4, H2O to 1 liter). In the last wash the eggs were suspended in S Basal (5.85 g NaCl, 1 g K2HPO4, 6 g KH2PO4, 1 ml cholesterol (5 mg/ml in ethanol), H2O to 1 liter), left under gentle agitation at 20°C for 16 h to be hatched the eggs. The L1 larvae were transferred to NGM agar with E. coli OP50 at 20°C for ~60 h until they reached the L4 stage [17]. After being collected and washed 3 times in S. Basal, they were centrifuged at (1300×g for 2 min) and the suspension was adjusted so that each 50μl contains ~400 L4 worms.
Experimental Design
The nematodes were transferred in triplicate for each reading, 50μl to each 96-wells plate containing 50μl of the milk samples and then incubated at 20°C. For each reading, at least 100 nematodes were recovered and analyzed by direct microscopy. To determine the survival rate of C. elegans, the number of live worms was recorded daily, and the percentage of surviving worms was calculated using the following formula: survival (%) = (live worms / total worms used) × 100. Worms were considered dead when they did not respond to being touched by a platinum wire pick.
Statistics Analysis
Study statistics were calculated using Stata/IC 15.0 software. Initially, the differences between the mean mortality rates of C. elegans when submitted to the milk of different groups of goats, as well as those of the control group, made up of samples of bovine milk, were verified. T-tests were performed for independent samples in 4 (four) sample groups. In those significant t-tests where equality of variance was not verified, the command was changed to “unequal”, since a requirement of the t-test is equality of variances. Next, a stepwise multiple linear regression was performed considering not only the breed of animals, but also information regarding age and tested markers. The absence of multicollinearity between the explanatory variables was verified using the VIF test, as well as the homoscedasticity of the residues using the white test. It was also investigated the normality of the residues, where although the normal distribution was not verified, because it was a relatively large sample, corrections were not necessary [18].
Results
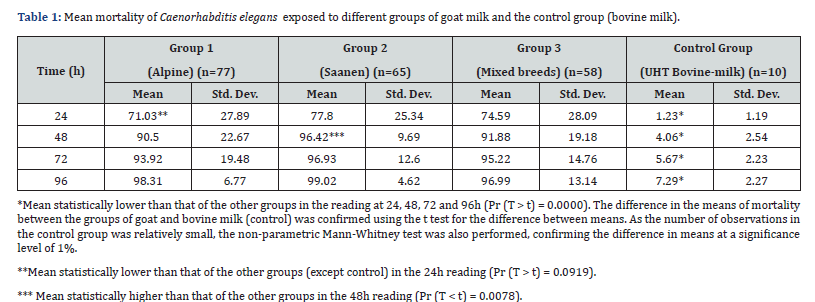
Our tests using Caenorhabditis elegans revealed that goat milk samples showed nematicidal efficacy compared to UHT bovine milk (p ≤ 0.05). In general, goat milk samples present different levels of such activity. There was a variation in the response in the first 24 hours, in which 7% of the samples were able to kill all exposed C. elegans, while 3% were not able to kill any worms. With an overall average mortality of 74% in worms exposed for 24 hours, 90% in 48 hours and 95% in 96 hours. In this study, 5% of the goats did not produce milk with considerable nematicidal activity, and their rates were compatible with the negative control.
The general averages of the animals of the Saanen breed were statistically the highest of the tested breeds in the 48-hour reading. Statistical significance was also observed in the 24-hour reading, where milk from Alpine goats showed a lower mortality rate when compared to the other two groups (Table 1). No correlation was observed with nematicity levels and age, SCC, and TBC. To confirm these results, a stepwise linear regression was executed to identify parameters that best correlate the variation in C. elegans mortality. Stepwise multiple linear regression uncovered the set of factors that best predict and possibly influences C. elegans mortality. In this case, animals from the Saanen group, when isolated, presented milk with a statistically higher level of mortality in the readings of 24 hours (p=0.071) and 48 hours (p=0.014) when compared to the other breeds and isolated parameters.
Discussion
As a nematode organism, C. elegans has biological characteristics common to other nematodes such as parasites, making it useful for pre-clinical research of nematicidal agents. In such cases, it can be said that C. elegans is an important primary platform for anthelmintic research, due to the difficulties of cultivating parasitic nematodes, mainly the need for a second host organism. Our analysis showed that it is possible to highlight milk samples with nematicidal activity using C. elegans. Milk from ruminants is being investigated as an antipathogenic agent for use as an enhancer or even as a substitute for classic allopathic drugs, where its use is especially interesting in organic livestock. Organic livestock is connected in several ways based on the One Health’s concept, being a production system based on the creation of animals as natural as possible, being economically viable and socially participative. Goat’s milk presents an excellent source of natural ingredients for different applications in food and in the pharmaceutical industry. Processing techniques on an industrial or semi-industrial scale are available for fractionation and isolation of the main milk proteins. Alimi and collaborators carried out the main work evaluating the nematicidal activity of raw milk and kefir produced through samples of several ruminates. In those tests the parasitic nematodes Haemonchus contortus and Heligmosomoides polygyrus were used [1,19]. Of the milk’s samples with nematicity activity tested, camel and goat were the ones that showed the best nematicidal activity, respectively.
Alimi and collaborators proceeded with the use of camel milk kefir as a nematicide agent in the control of H. polygyrus in infected rats, managing to demonstrate high levels of enteroparasites biological control. In these studies, was analyzed goat milk, but few animals’ milks were used, which called our attention to verify how the nematicidal activity would present itself in a large group of animals [2,19]. Despite the mortality of C. elegans when exposed to the milk of Saanen goats were statistically higher in our analysis. was possible to select samples with high nematicidal capacity in all goat breeds milk’s tested, making them all interesting for this purpose. The goat’s ages did not show significant differences in our tests. This could be considered beneficial, considering milk as an industrial input. Not having to discard older animals and already being able to use the milk of young animals is advantageous for producers. The main markers of mastitis in milk, SCC and TBC, also showed no correlation with C. elegans mortality rates. Mastitis is directly linked to multi-drug resistant agents, these agents are a threatening problem for the livestock industry, which represents a major threat to the general well-being and livestock production in the world. The most significant factors in multi-drug resistant agents’ incidence are an excessive frequency of treatments and the administration of an inadequate dose (underdosage), where these problems are particularly prominent in developing countries. The use of milk as an antipathogenic agent can benefit animals with other diseases such as mastitis due to the antimicrobial and anti-inflammatory activities already evidenced in this compound. Production animals, such as goats, may benefit from the use of milk as a nematicidal agent, as they present a high incidence of gastrointestinal parasitism, with the use of anthelmintics being the main therapeutic strategy for parasite control in those animals [20]. Thus, it allows the possibility of overcoming multi resistance using natural compounds that offer low cost and accessibility.
Studies that evaluate which animals produce milk with the best nematicide potential have great biotechnological value, as well as research that investigates this same potential in other animals not yet described, such as mares, buffaloes, and donkeys. It is important in future research to correlate the nematicity of the milk of these ruminants with its constituents, with the hormonal and immunological status of the animal and how this activity varies seasonally to be able to track which fractions have the greatest potential for application. As the active molecules involved in this activity are not yet known, further studies are needed to elucidate these molecular processes and to evaluate the capacity of milk control enteroparasites in different infected animals.
Conclusions
As far as we know, this is the first time that C. elegans was used to access the nematicide activity of raw milk. It was possible to locate samples with high nematicidal activity in all goat’s breed and age tested, which makes them all interested in using their milk. It is an important finding, since this activity varies between animals and until we know better how this nematicity presents itself at different times and their molecular bases, it is necessary to verify this activity for an efficient use.
Acknowledgment
This research was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Fundação de Amparo à Pesquisa do Estado de Minas Gerais (Fapemig) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (Capes) Finance code 001.
References
- Alimi D (2016) First Report of The In Vitro Nematicidal Effects of Camel Milk. Veterinary Parasitology 228: 153-159.
- Alimi D (2018) In Vivo Nematicidal Potential of Camel Milk on Heligmosomoides Polygyrus Gastro-Intestinal Nematode of Rodents. Helminthologia 55(2): 112-118.
- Atanasova J, Ivanova I (2010) Antibacterial Peptides from Goat and Sheep Milk Proteins. Biotechnology & Biotechnological Equipment 24(2): 1799-1803.
- Azizkhani M, Saris Pej, Baniasadi M (2021) An In-Vitro Assessment of Antifungal and Antibacterial Activity of Cow, Camel, Ewe, And Goat Milk Kefir and Probiotic Yogurt. Journal of Food Measurement and Characterization 15(1): 406-415.
- Biadała A (2020) Antimicrobial Activity of Goat’s Milk Fermented by Single Strain of Kefir Grain Microflora. European Food Research and Technology 246(6): 1231-1239.
- Luz C (2020) Antifungal and Antimycotoxigenic Activity of Hydrolyzed Goat Whey on Penicillium SPP: An Application as Bio preservation Agent in Pita Bread. LWT 118: 108717.
- Zanutto-Elgui MR (2019) Production of Milk Peptides with Antimicrobial and Antioxidant Properties Through Fungal Proteases. Food Chemistry 278: 823-831.
- FAO (2016) Thinking About the Future of Food Safety. FAO.
- Instituto Brasileiro De Geografia E Estatística – Ibge (2019) Sistema Ibge De Recuperaçao Automatica´-Sidra.
- Pilarczyk B (2021) Comparison of The Prevalence of The Parasites of The Digestive Tract in Goats from Organic and Conventional Farms. Animals (Basel) 1: 2581.
- Adduci I (2022) Haemonchosis in Sheep and Goats, Control Strategies and Development of Vaccines Against Haemonchus Contortus. Animals 12(18): 2339
- Keiser J, Utzinger J (2010) The Drugs We Have and The Drugs we need Against Major Helminth Infections. Advances in Parasitology. Elsevier 73: 197-230.
- Pancu DF (2021) Antibiotics: Conventional Therapy and Natural Compounds with Antibacterial Activity-A Pharmaco-Toxicological Screening. Antibiotics 10(4): 401-407.
- Burns AR (2015) Caenorhabditis Elegans is a Useful Model for Anthelmintic Discovery. Nature Communications 6(1): 7485.
- Hahnel SR (2020) Caenorhabditis Elegans in Anthelmintic Research – Old Model, New Perspectives. International Journal for Parasitology: Drugs and Drug Resistance 14: 237-248.
- Stiernagle T (2006) Maintenance of C. Elegans. Worm book.
- Porta-De-La-Riva M (2012) Basic Caenorhabditis Elegans Methods: Synchronization and Observation. Journal of Visualized Experiments 64: 4019.
- Hair Junior (2009) Análise Multivariada De Dados. (6a (Ed)). Porto Alegre: Bookman. Porto Alegre: Bookman.
- Alimi D, Rekik M, Akkari H (2019) Comparative In Vitro Efficacy of Kefir Produced from Camel, Goat, Ewe And Cow Milk On Haemonchus Contortus. Journal of Helminthology 93(4): 440-446.
- Mpofu TJ, Nephawe KA, Mtileni B (2022) Prevalence and Resistance to Gastrointestinal Parasites in Goats: A Review. Veterinary World 15(10): 2442-2452.






























